CIE A Level Chemistry复习笔记2.1.6 Period 3 Elements: Electronegativity & Bonding
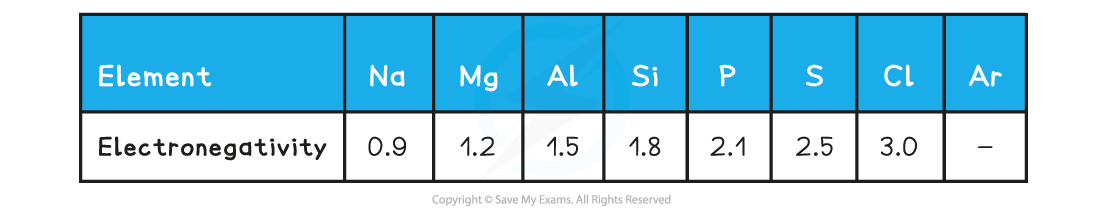
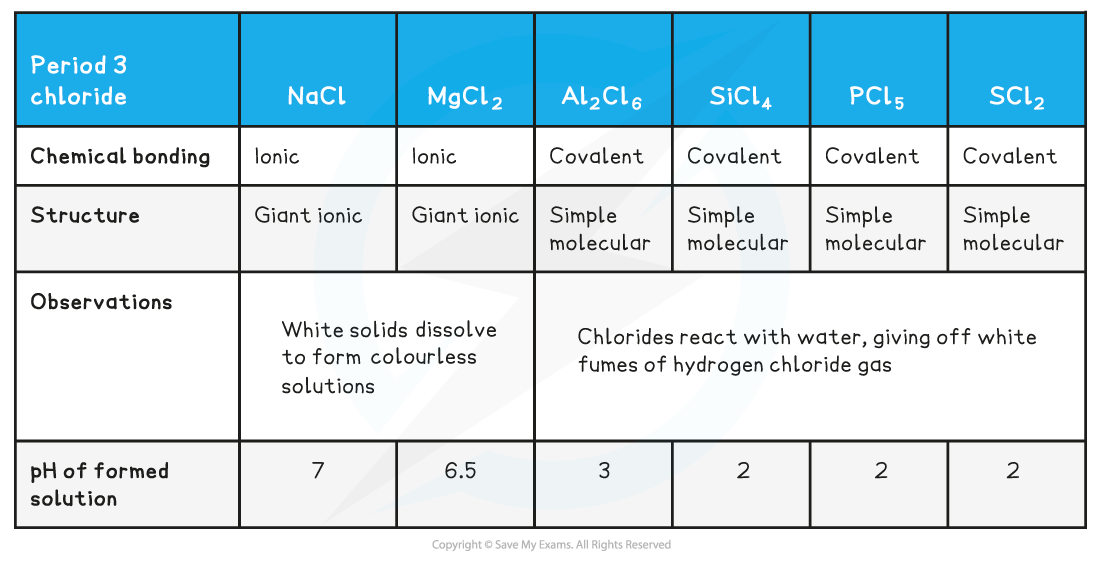
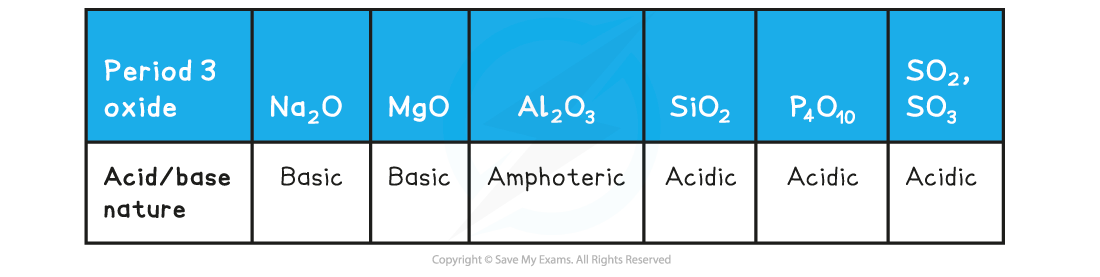
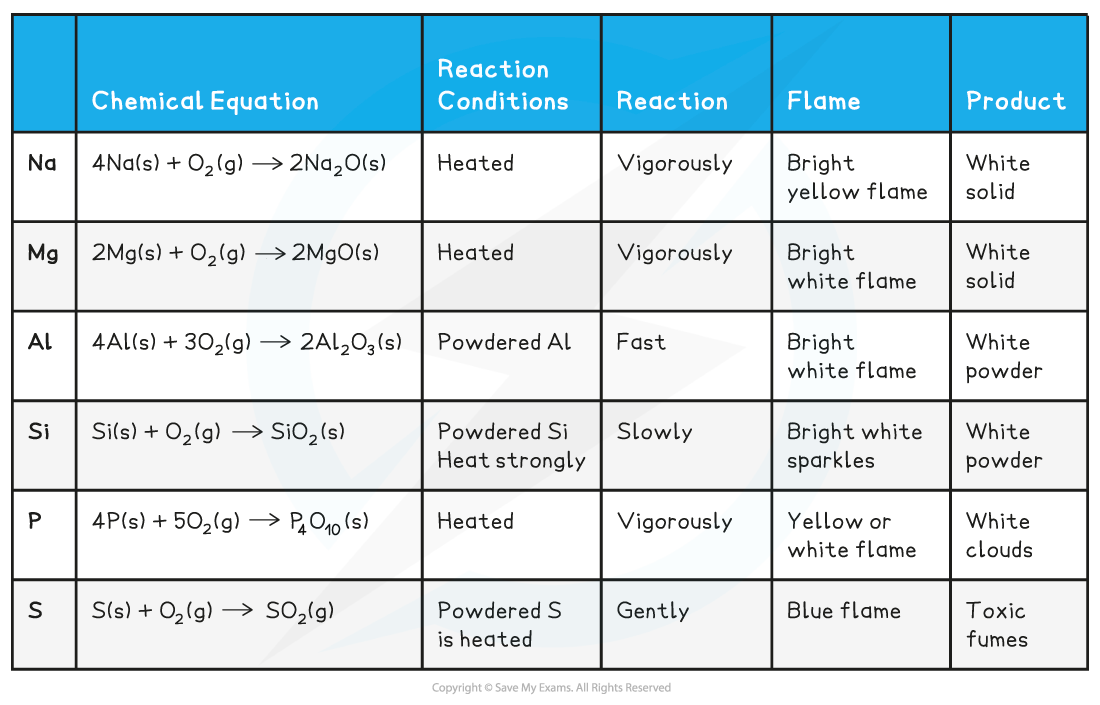
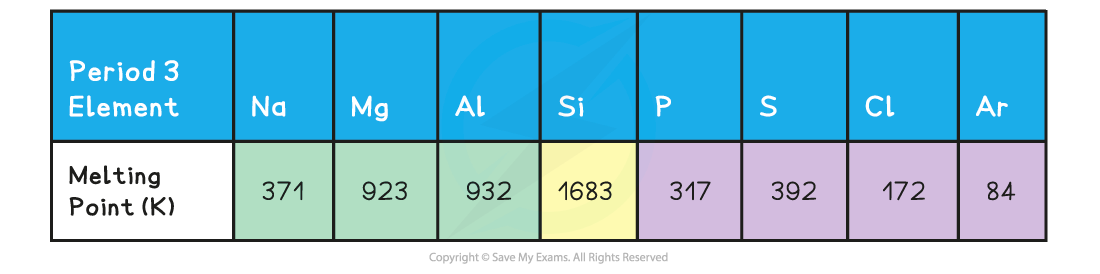
Period 3: Trends in Electronegativity & Bonding Electronegativity Electronegativity is the power of an element to draw the electrons towards itself in a covalent bond Going across the period, t...