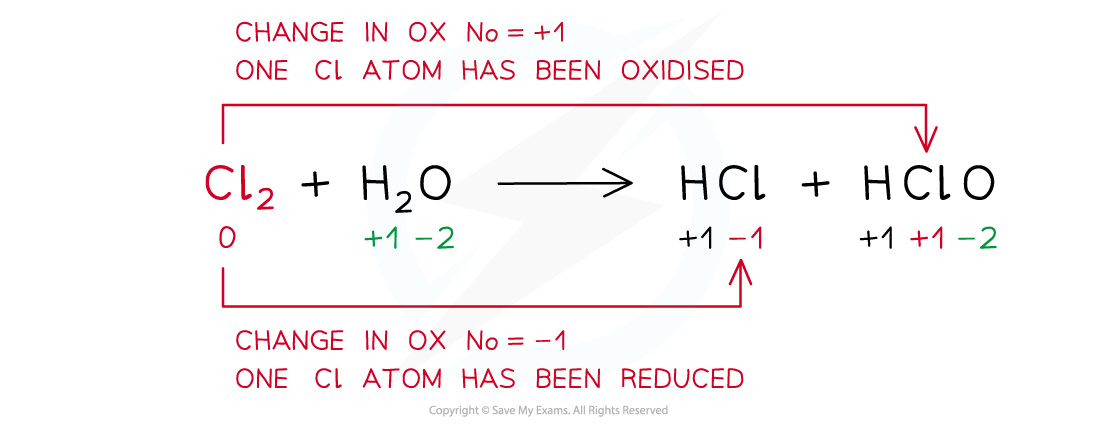
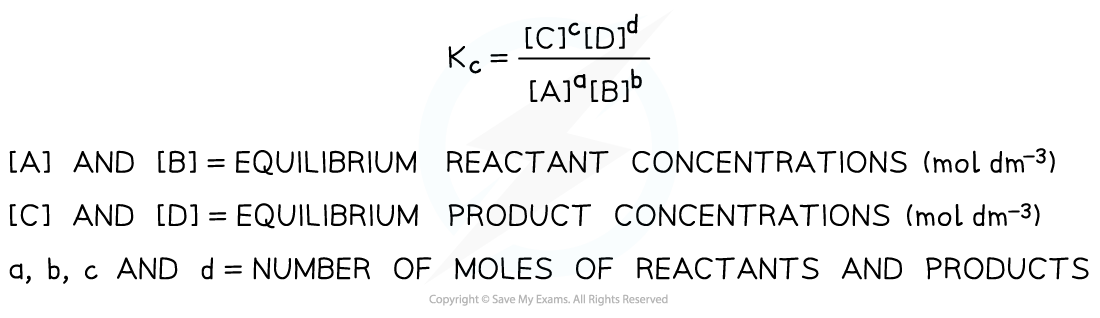
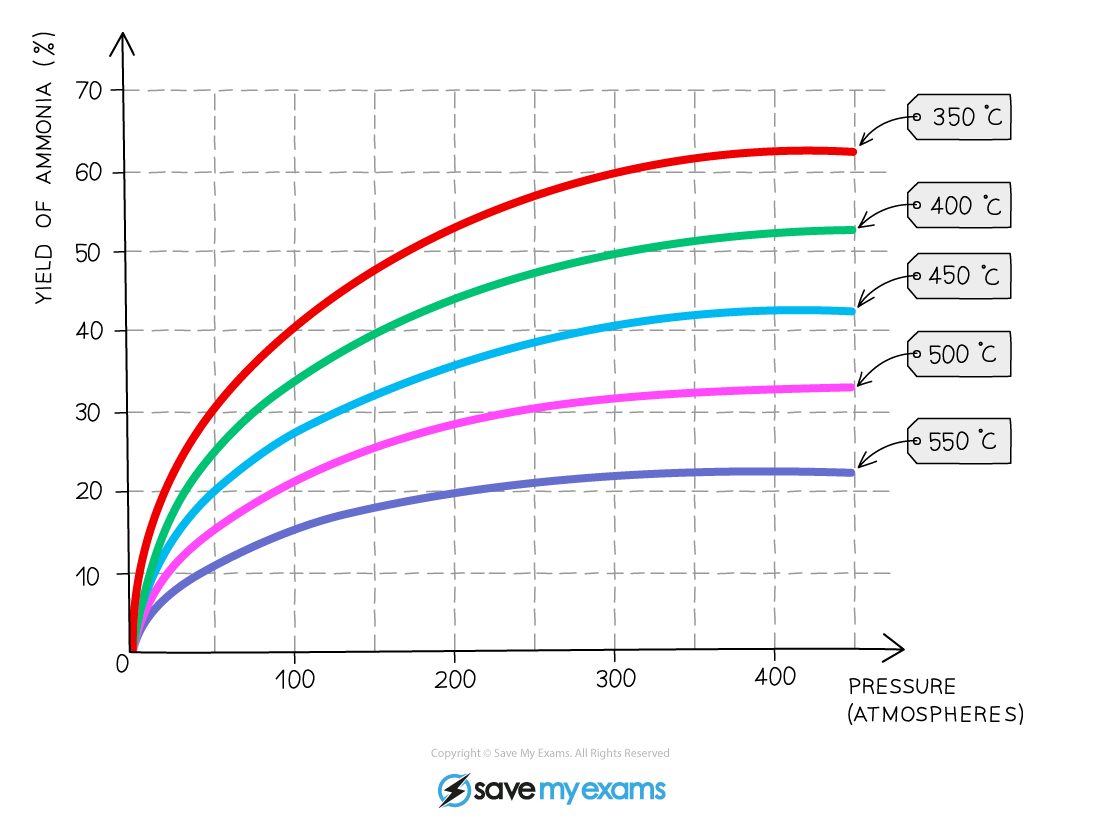
Edexcel A Level Chemistry:复习笔记2.2.1 Explaining Group 2 Trends
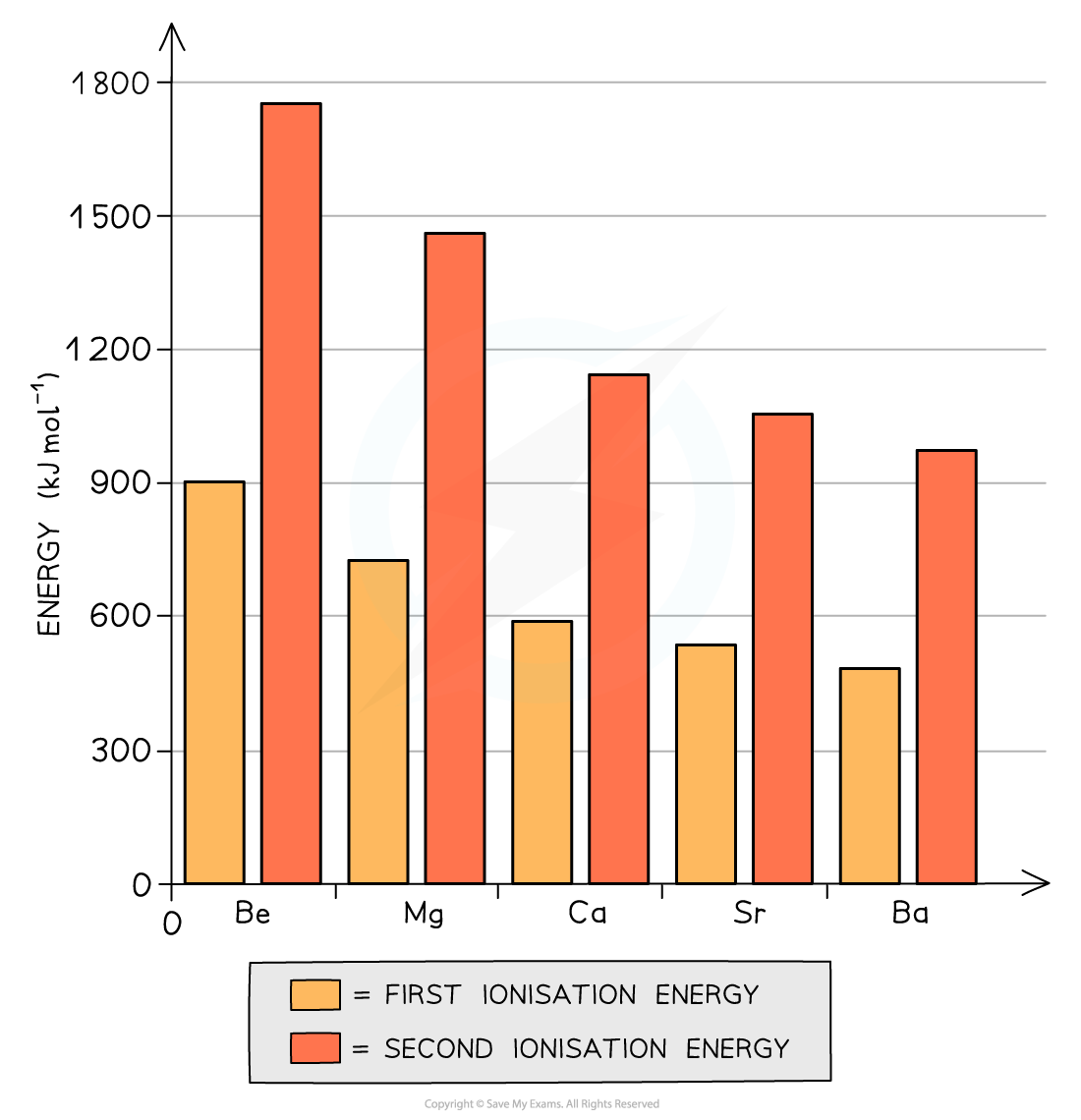
Ionisation Energy Chemical trends All elements in Group 2 (also called alkali earth metals) have two electrons in their outermost principal quantum shell All Group 2 metals can form ionic co...