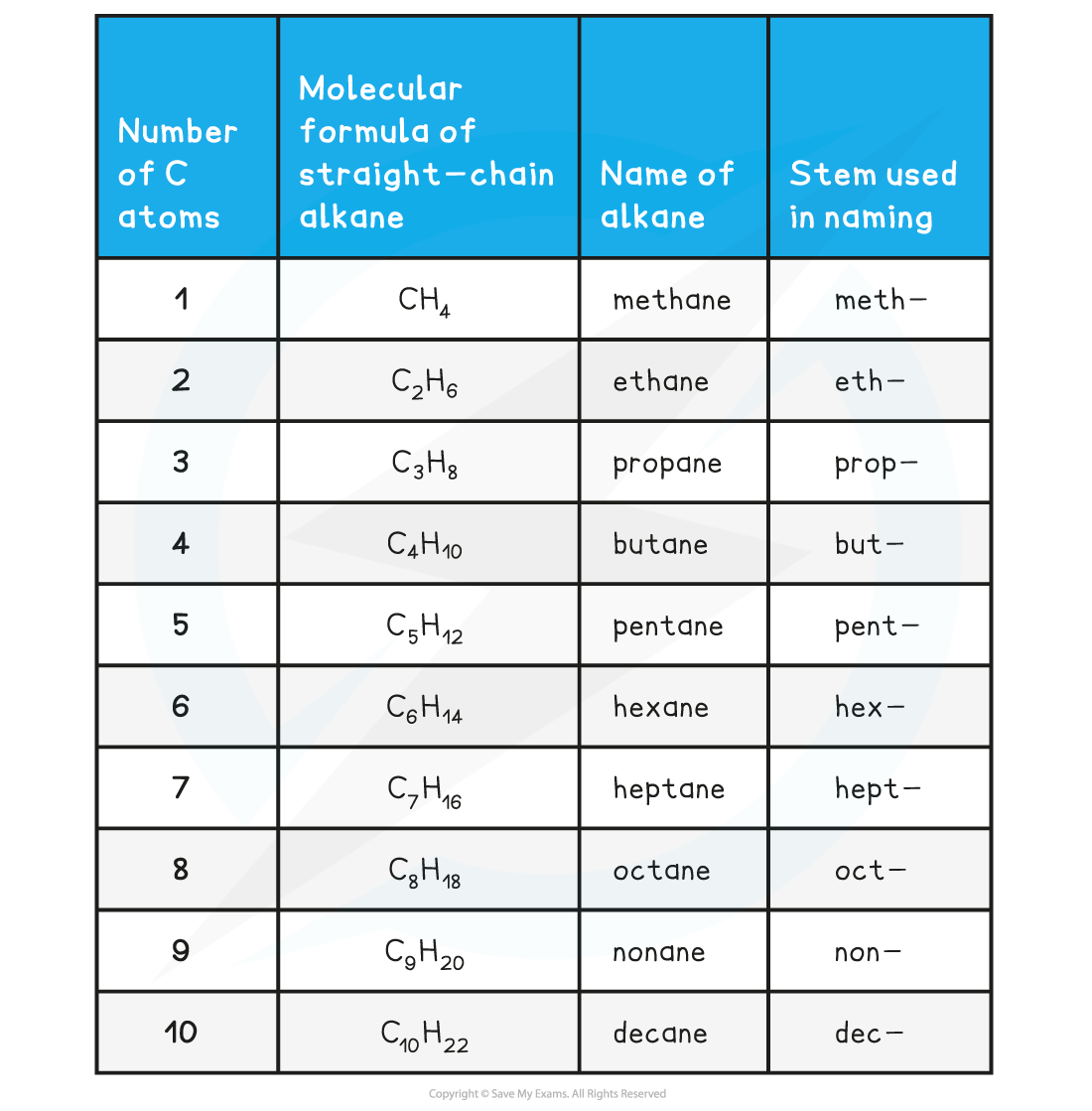
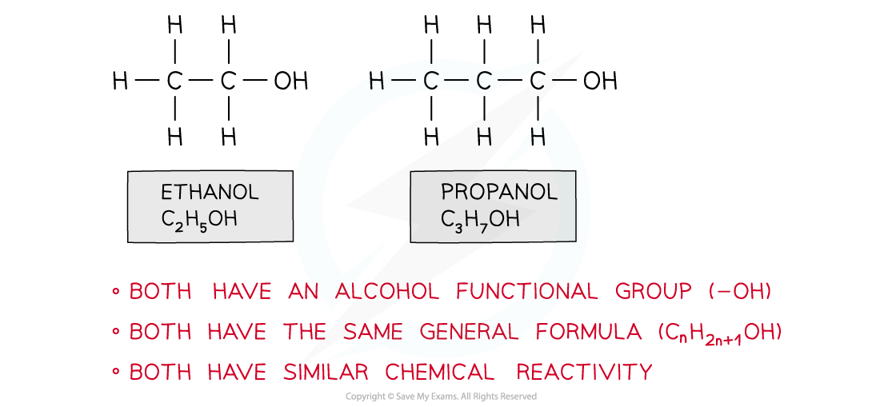
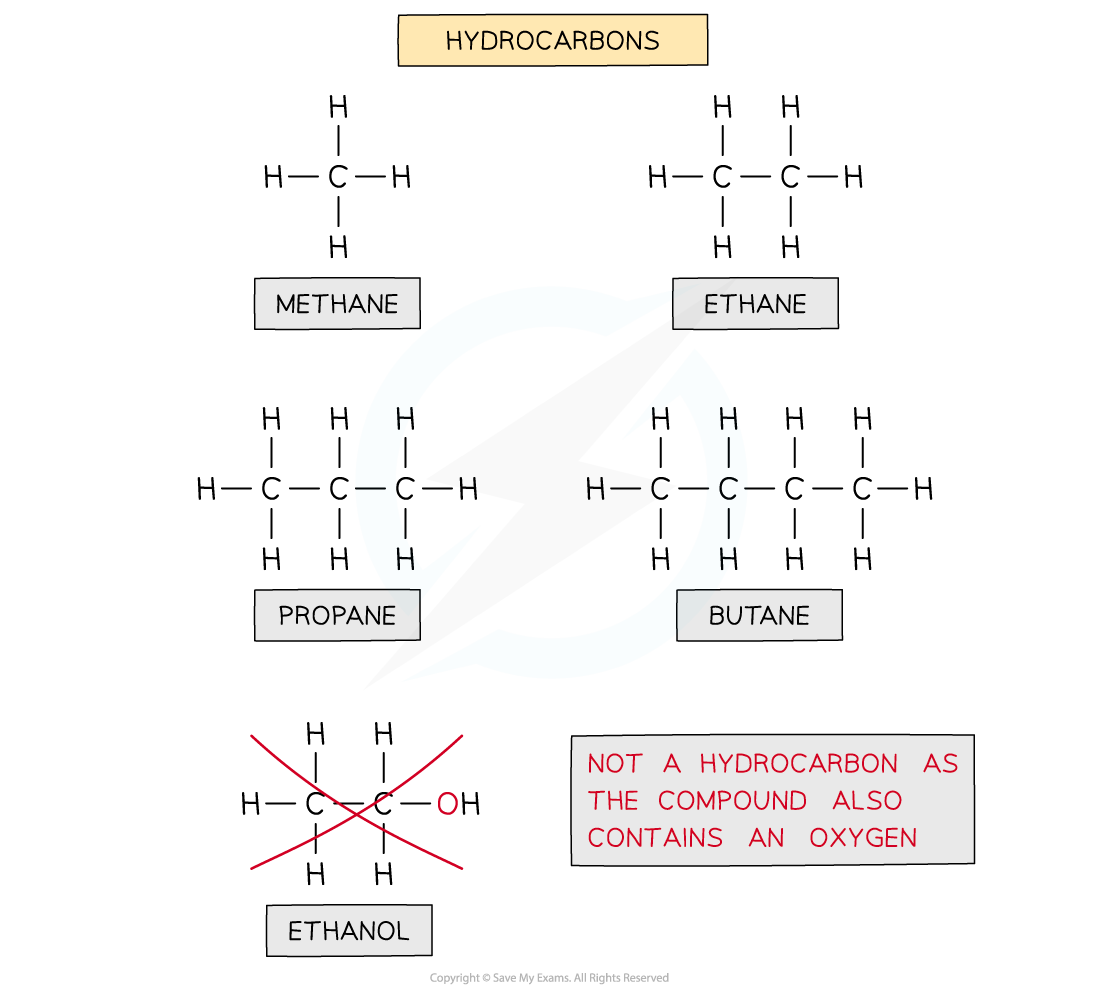
Edexcel A Level Chemistry:复习笔记3.2.3 Pollution from Combustion
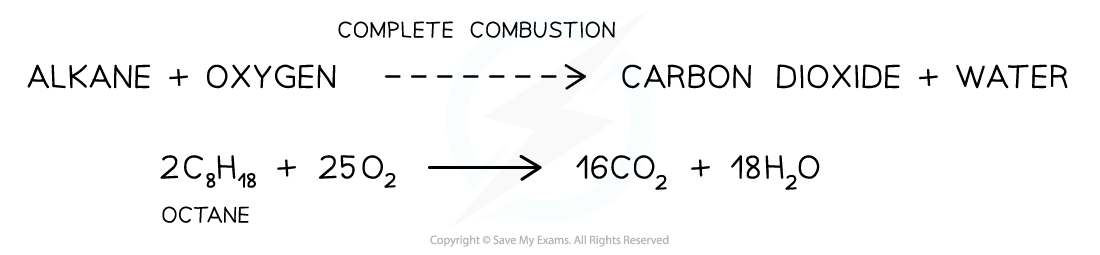
Causes & Effects of Pollution Alkanes are combusted (burnt) on a large scale for their use as fuels Complete combustion When alkanes are burnt in excess (plenty of) oxygen, complete comb...