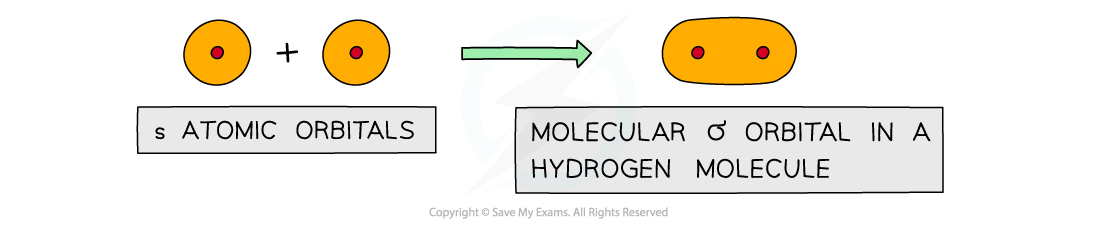
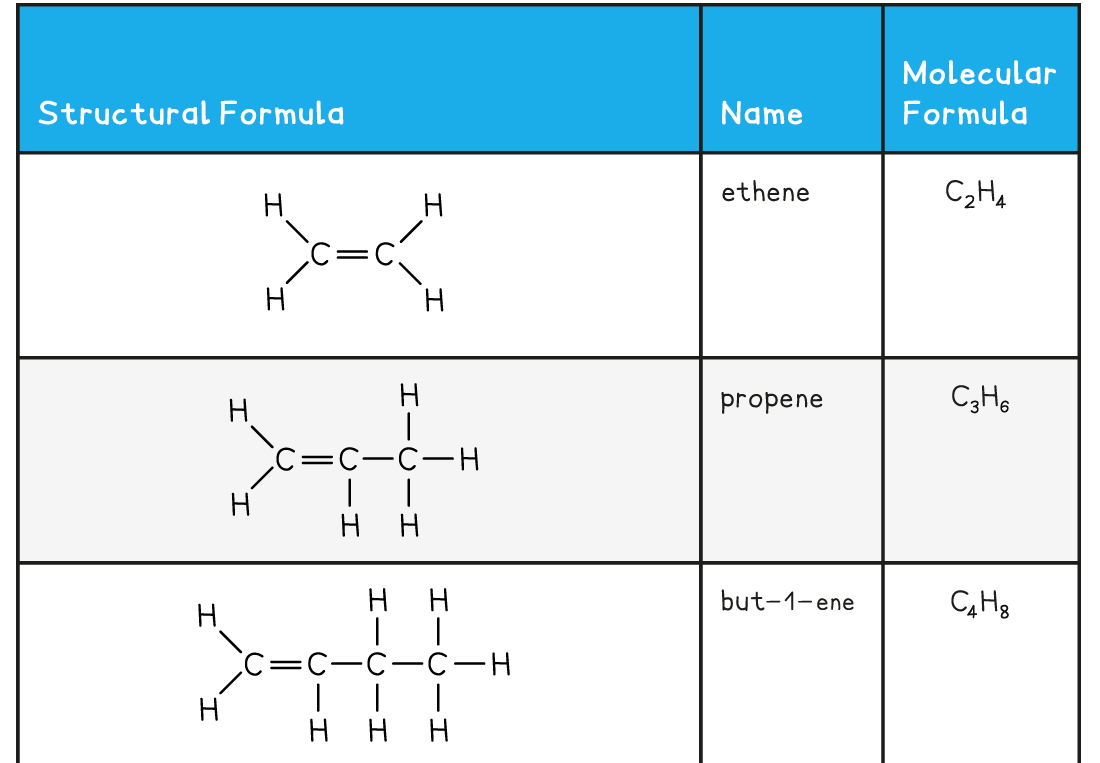
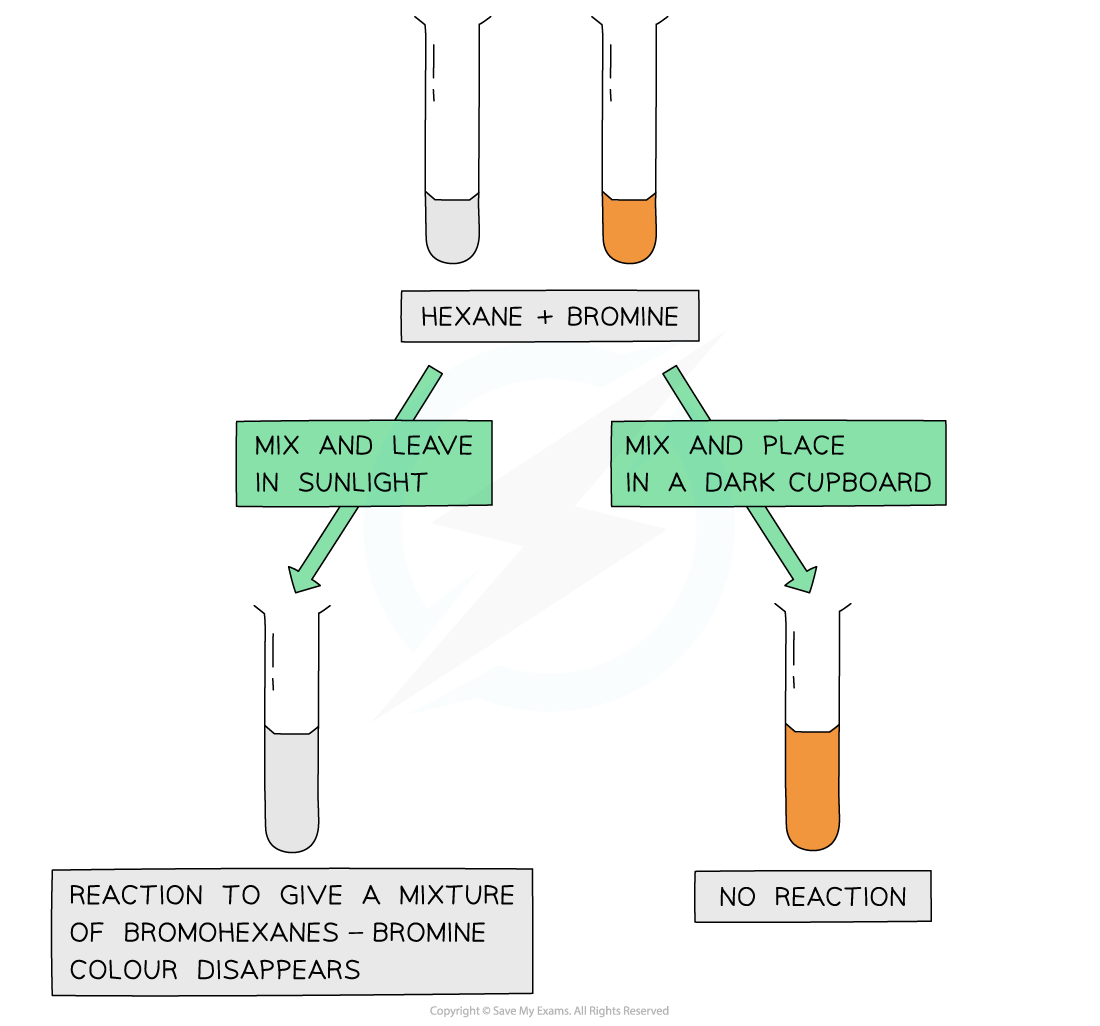
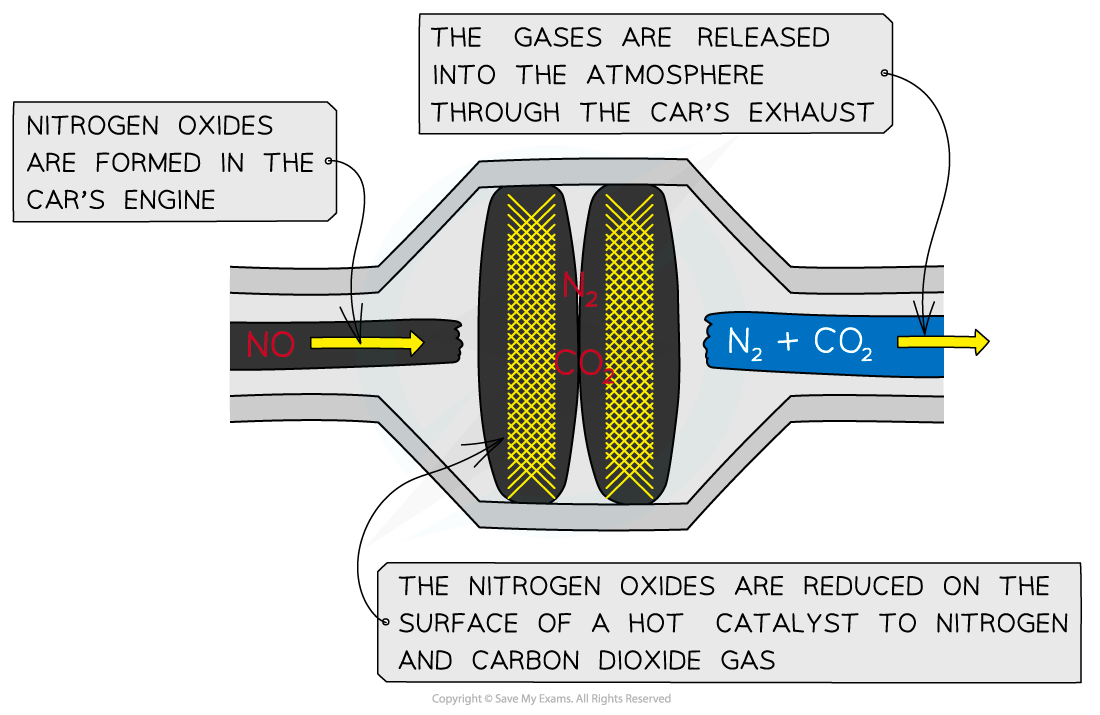
Edexcel A Level Chemistry:复习笔记3.3.6 Addition Polymerisation
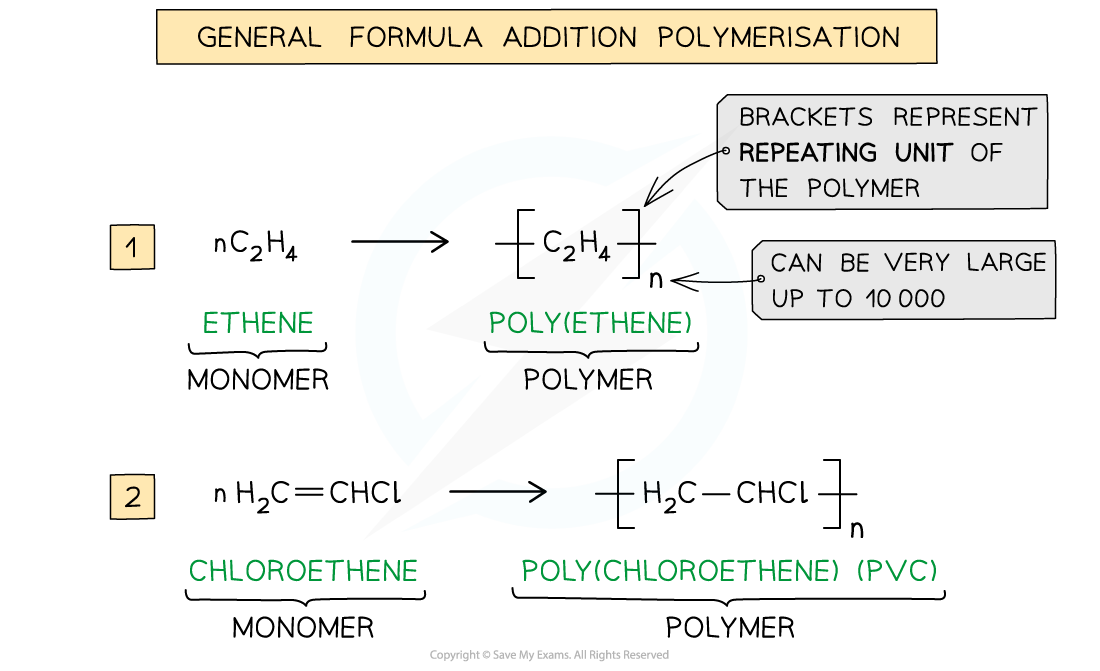
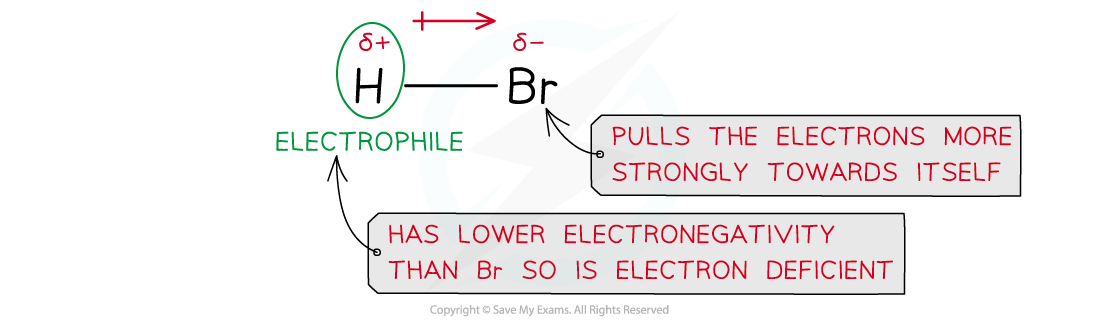
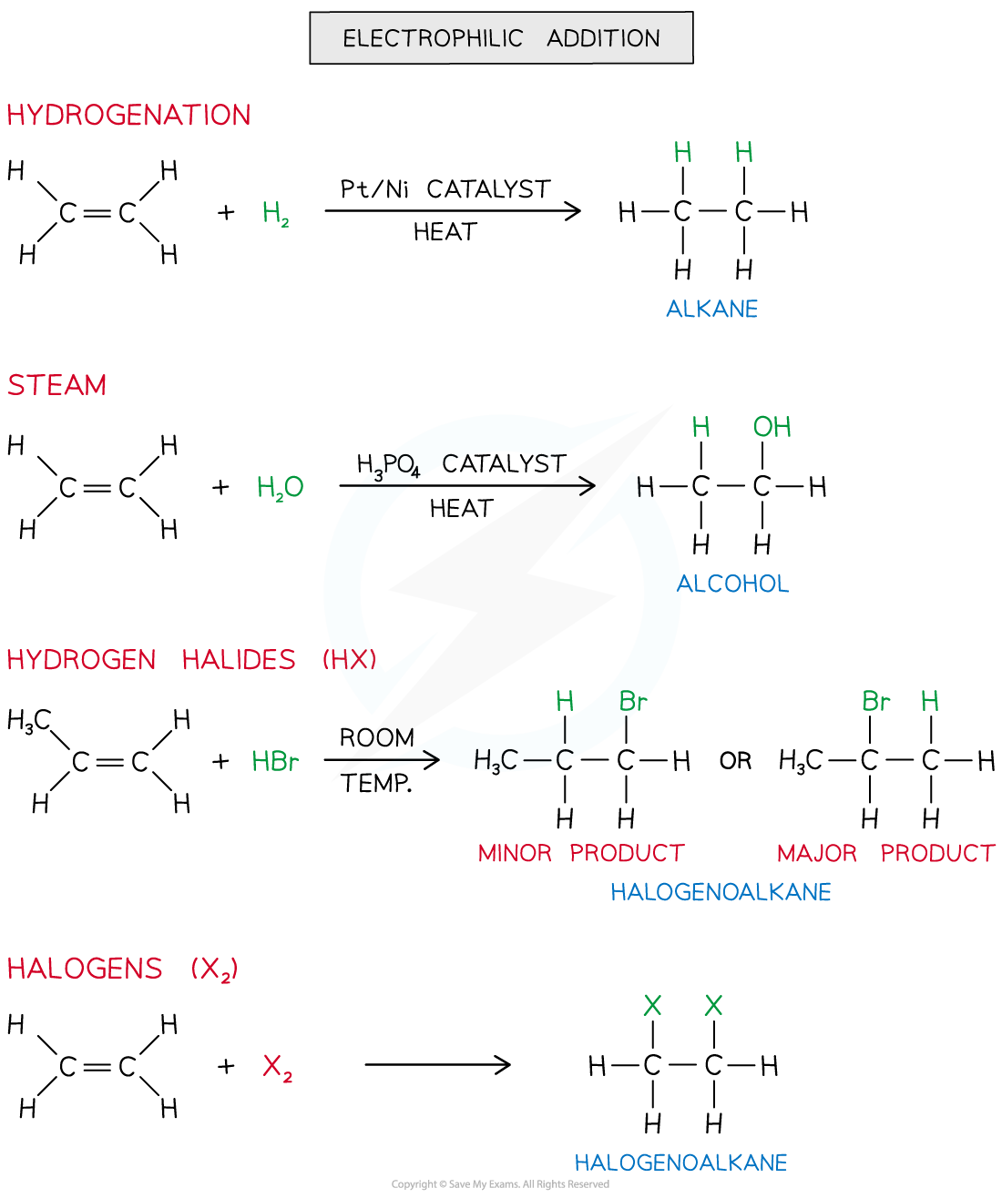
Addition Polymerisation Addition polymerisation Addition polymerisation is one of the most important addition reactions of alkenes which form the basis of the plastics industry Addition poly...