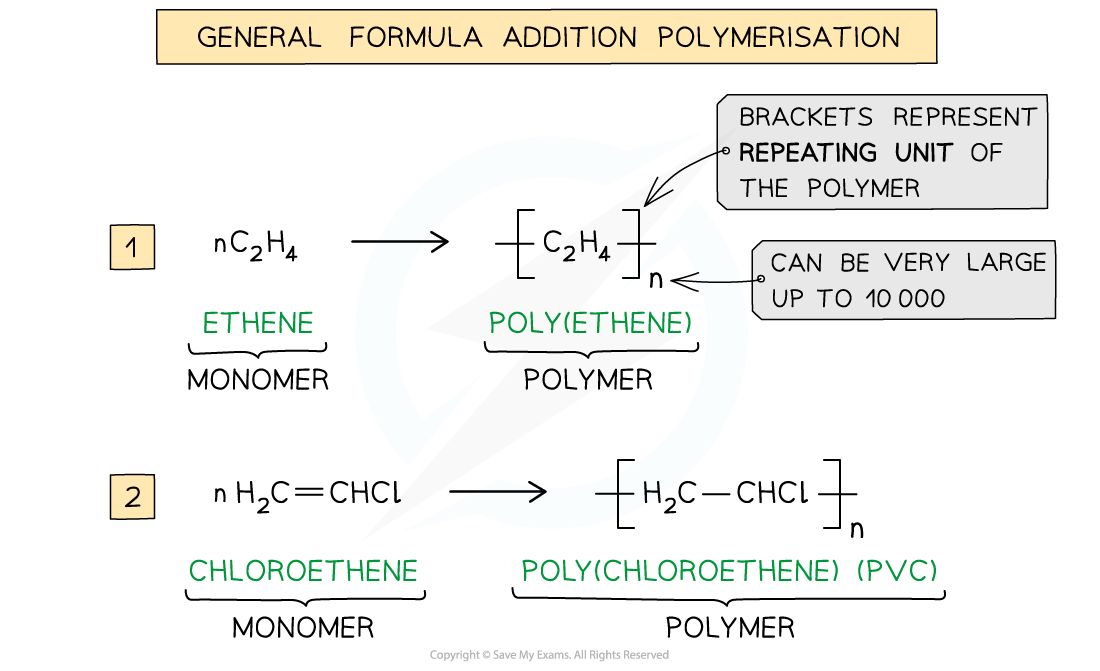
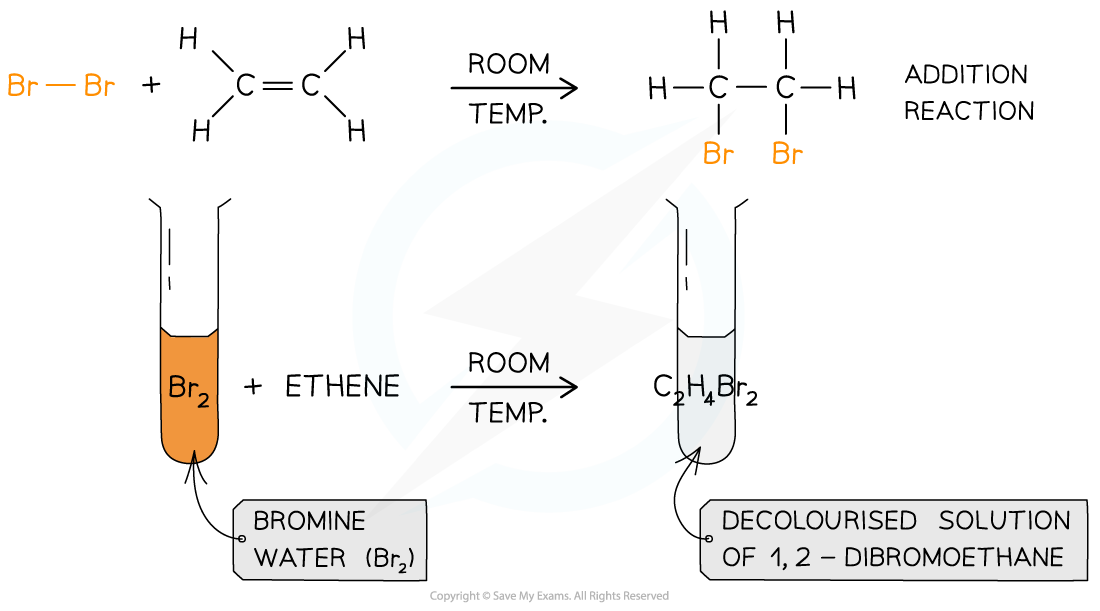
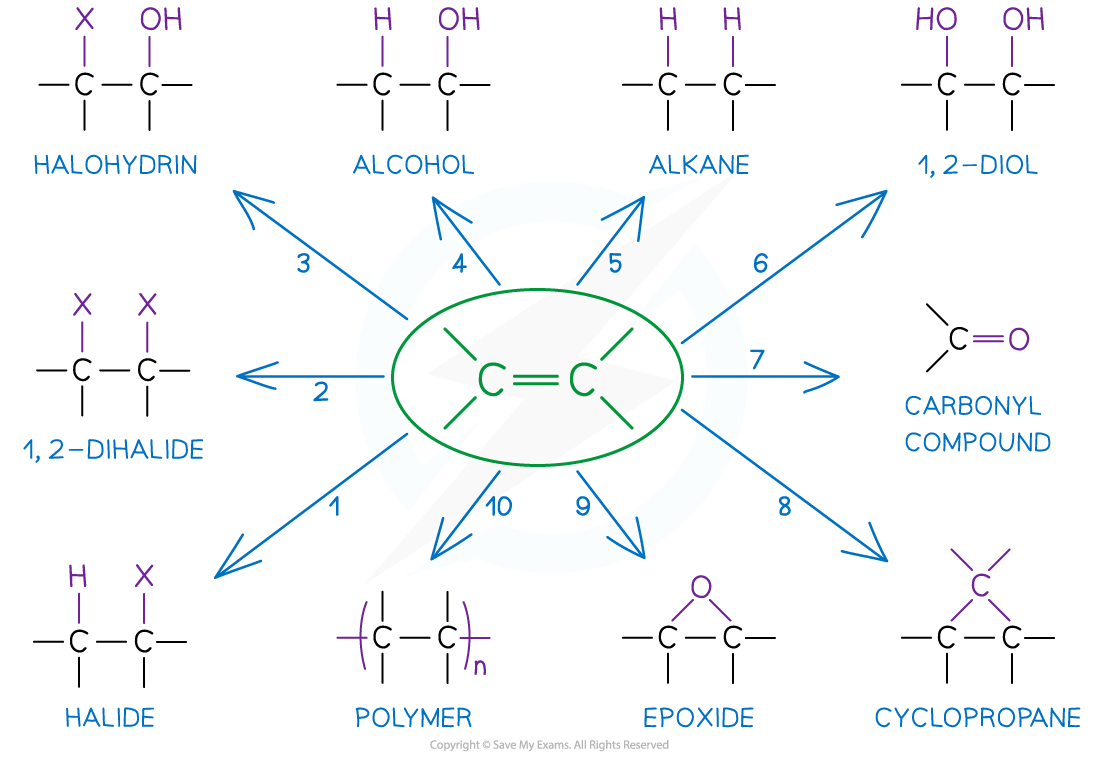
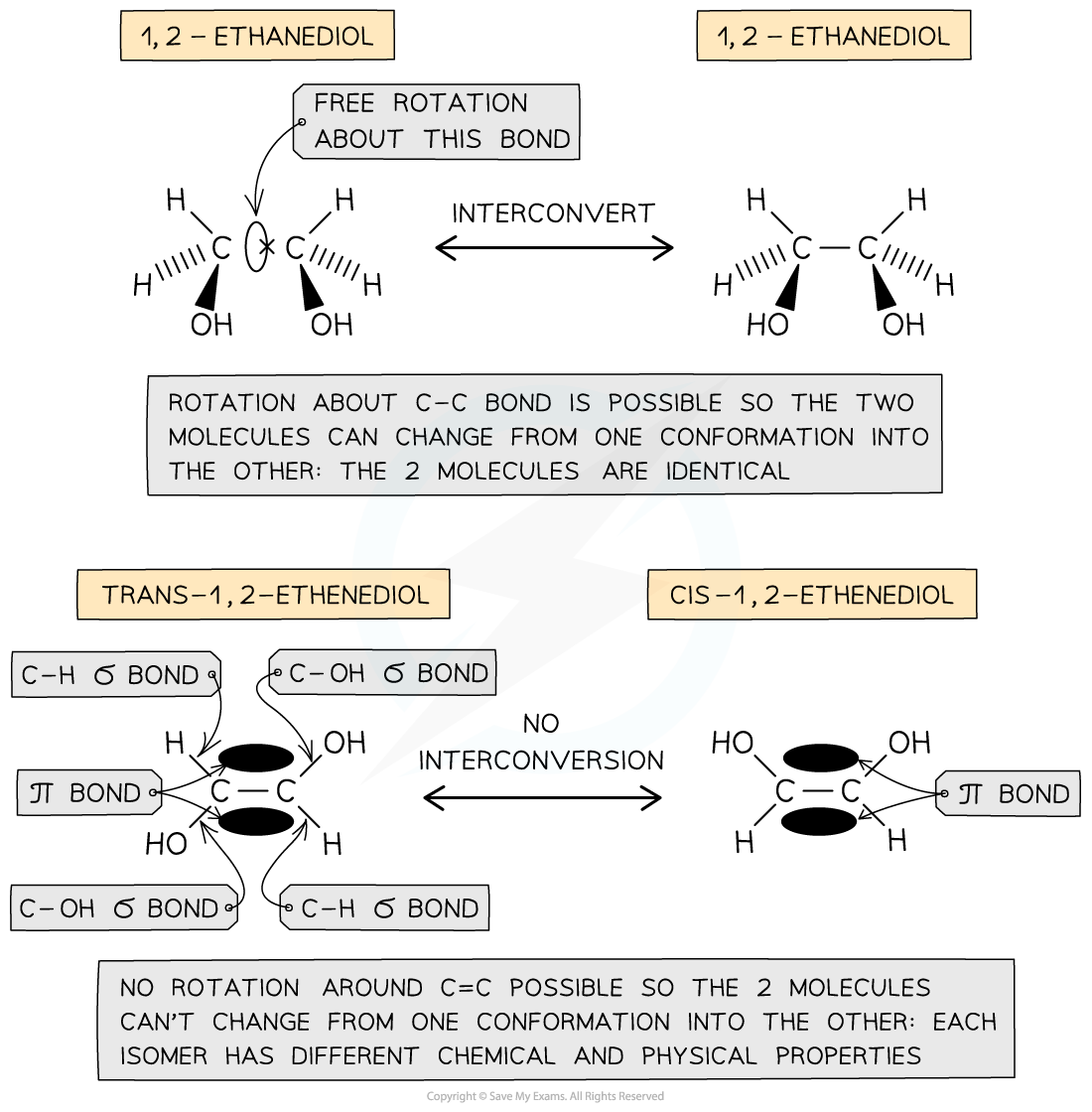
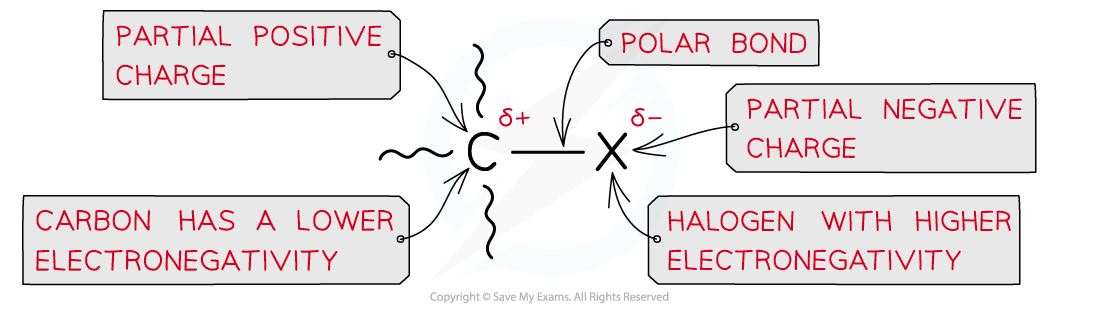
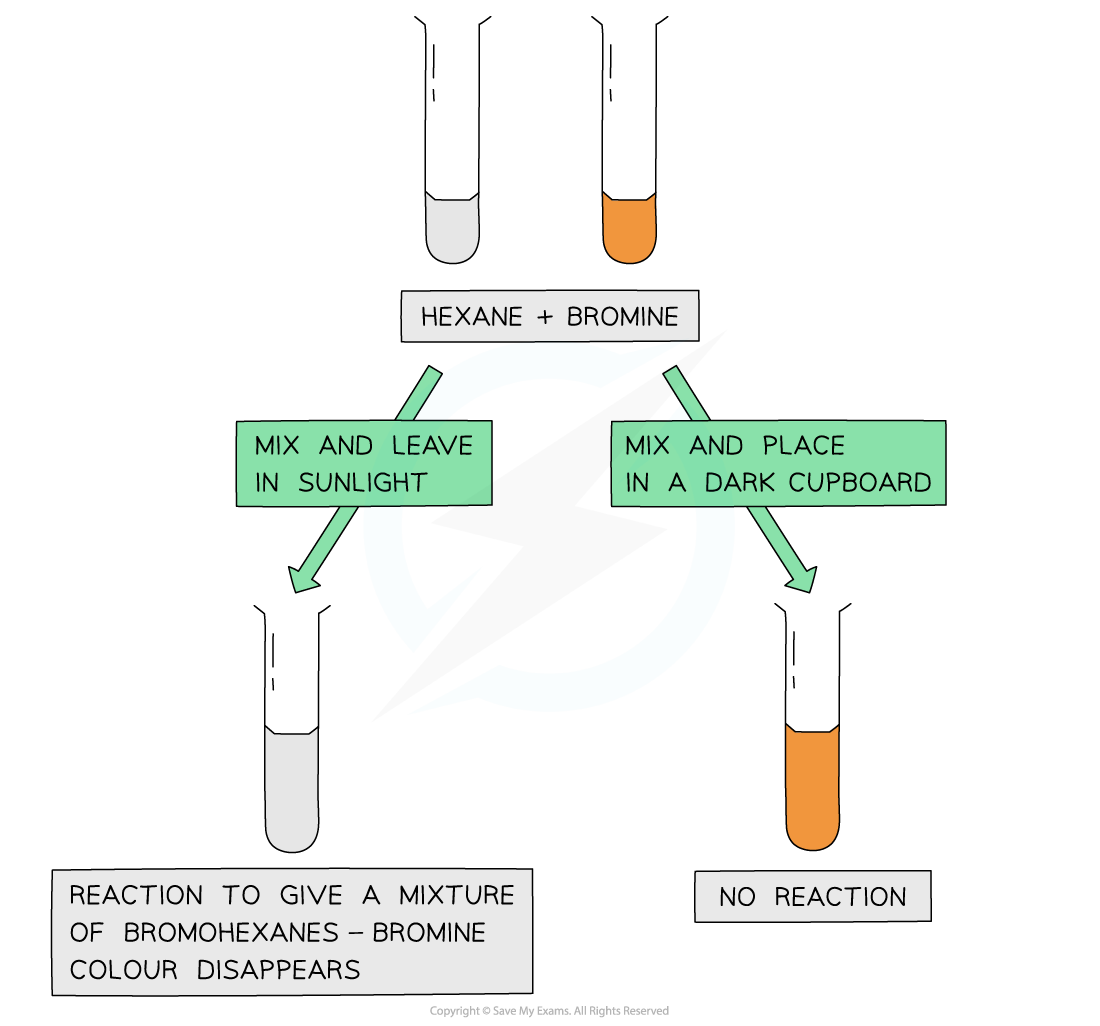
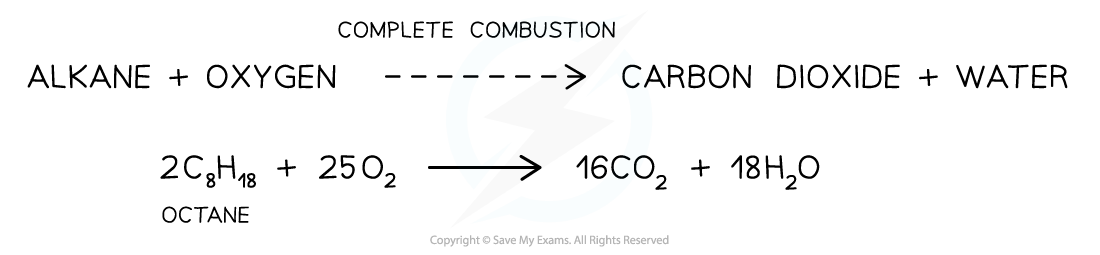
AQA A Level Chemistry复习笔记3.4.6 Solution to Plastic Pollution
Disposing of Polymers Though poly(alkenes)s are extremely important in everyday such as their use as plastics, the disposal of these polymers is problematic Poly(alkenes) are very large alka...