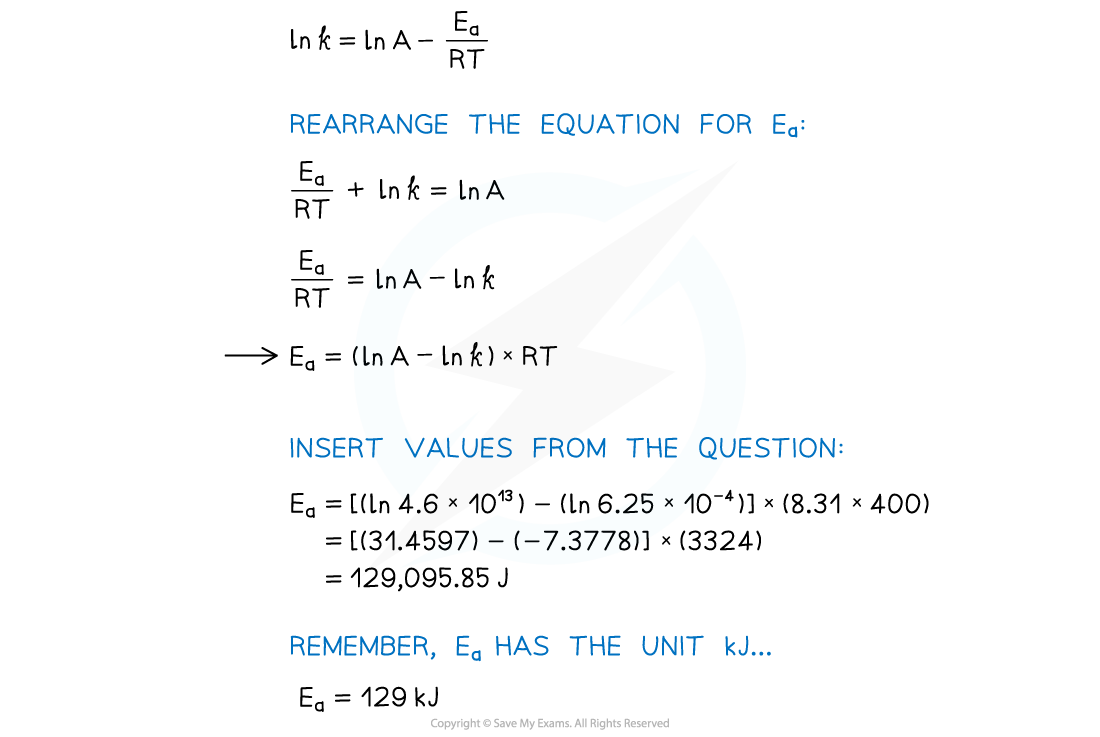
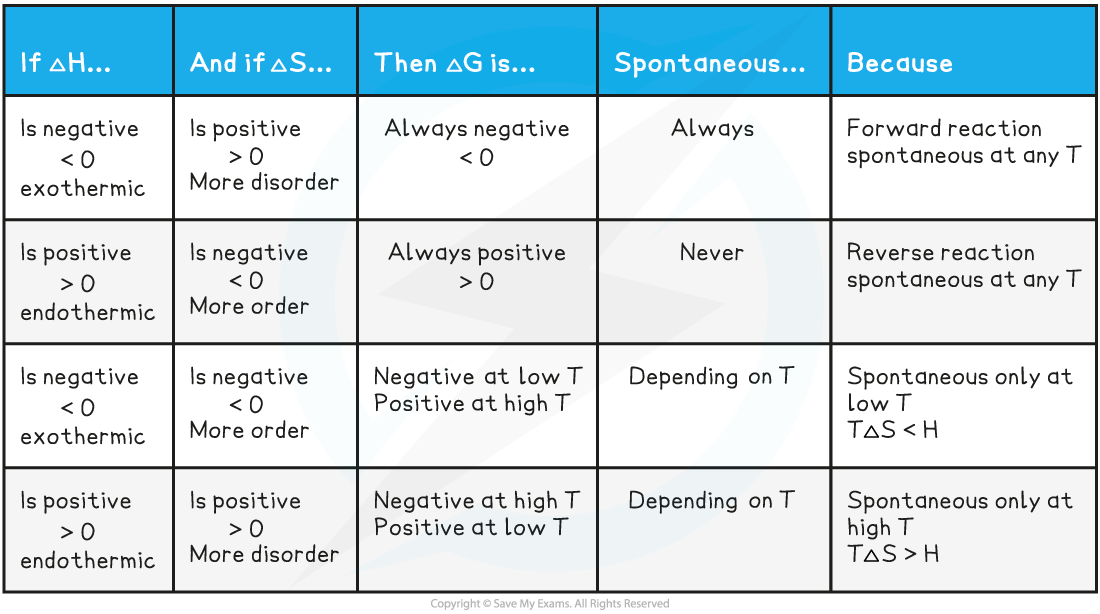
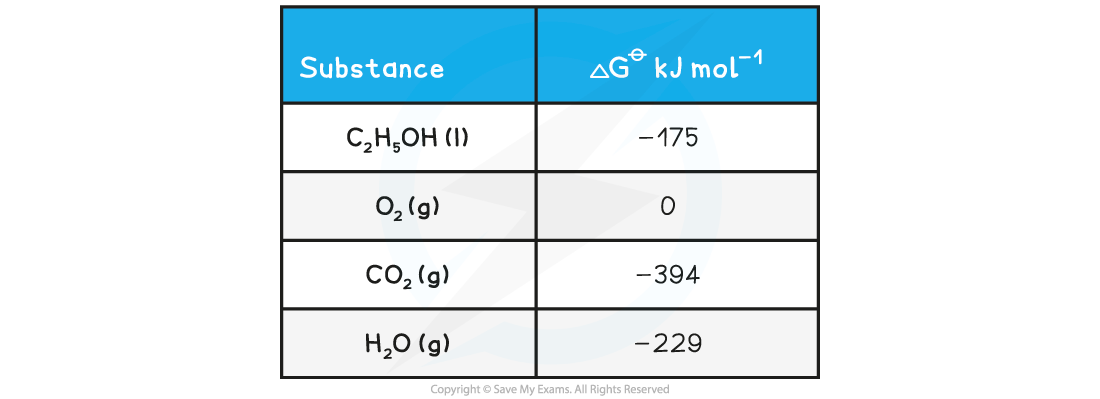
AQA A Level Chemistry复习笔记5.2.6 Concentration-Time Graphs
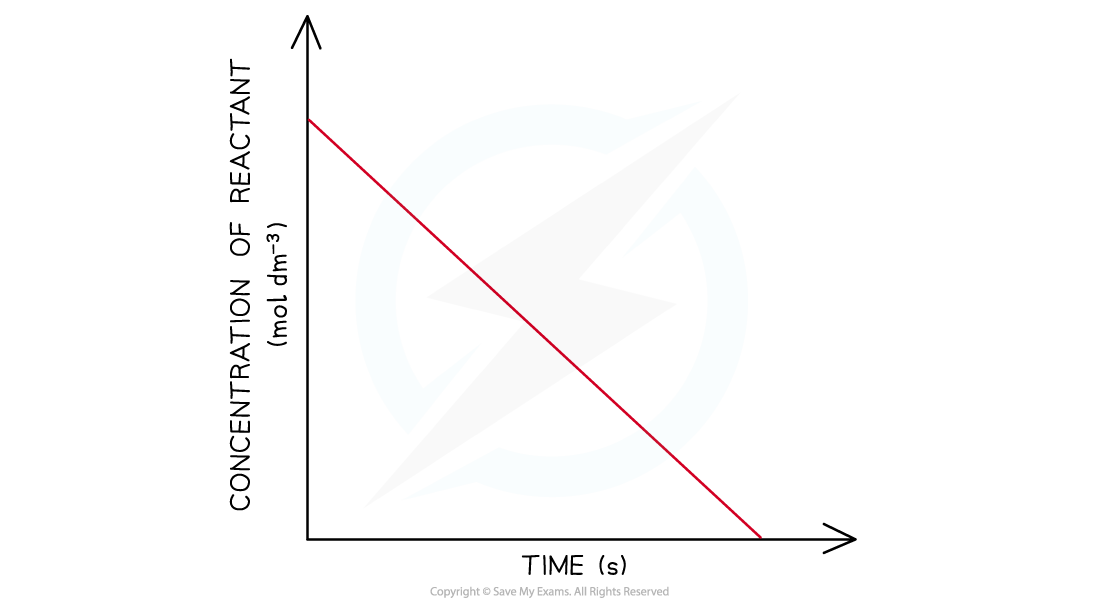
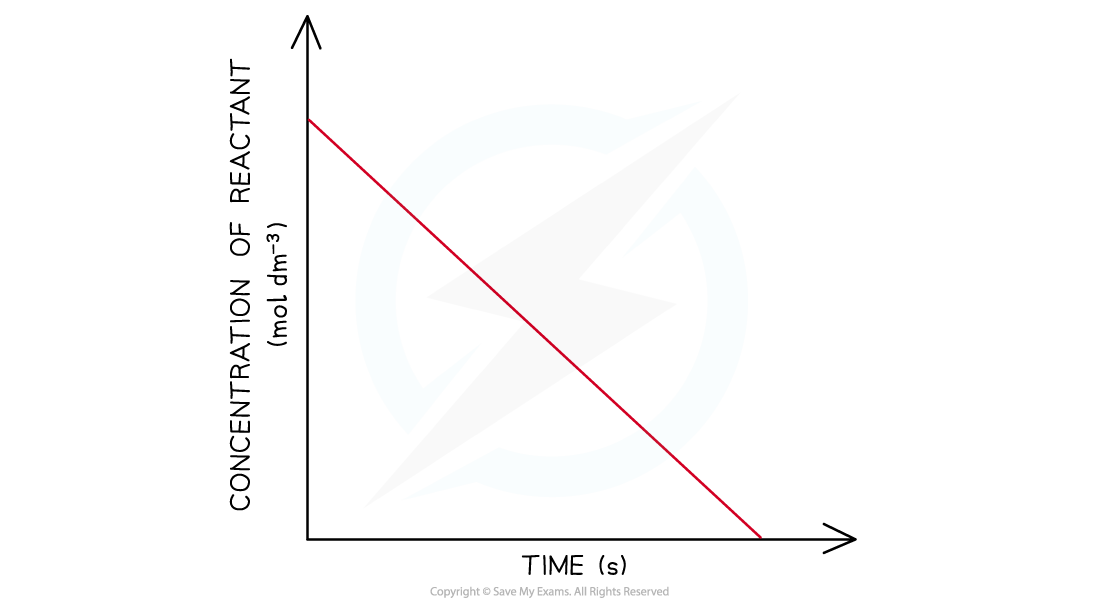
Concentration-Time Graphs Order of reaction from concentration-time graphs In a zero-order the concentration of the reactant is inversely proportional to time This means that the concentrati...