- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
2013 AP Chemistry化学真题系列之选择题免费下载
历年AP Calculus BC微积分BC系列
真题与答案下载

翰林国际教育全网首发
力争超快速发布最全资料
助你在升学路上一帆风顺
为你的未来保驾护航
2013 AP Chemistry Practice Exam Multiple Choice Free Download
2013 AP 化学模考选择题部分免费下载
此套Section I试卷共分计时1小时30分钟组成
共60题
仅限使用铅笔,无计算器
考试时会提供花常用的等式与常量
以及化学元素周期表
完整版下载链接见文末
部分真题预览:
1)Complete combustion of a sample of a hydrocarbon in excess oxygen produces equimolar quantities of carbon dioxide and water. Which of the following could be the molecular formula of the compound?
C2H2
C2H6
C4H8
C6H6
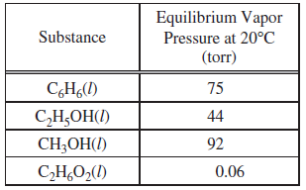
2)Based on the data in the table above, which of the following liquid substances has the weakest intermolecular forces?
C6H6(l)
C2H5OH(l)
CH3OH(l)
C2H6O2(l)

3)Based on the data in the table above, which of the following correctly predicts the relative strength of the attraction of Zn2+, Ca2+, and Ba2+ ions to water molecules in a solution, from strongest to weakest, and provides the correct reason?
Zn2+ > Ca2+ > Ba2+ because the smaller ions have a stronger coulombic attraction to water
Zn2+ > Ca2+ > Ba2+ because the smaller ions are more electronegative
Ba2+ > Ca2+ > Zn2+ because the larger ions are more polarizable
Ba2+ > Ca2+ > Zn2+ because the larger ions are less electronegative
4)Zn(s) is used to reduce other compounds in chemical reactions. If a chemist needs a substance that is more effective in its reducing ability, which of the following species would be the best choice?
Na
H+
K+
Cl−
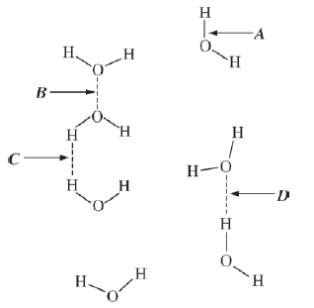
5) In the diagram above, which of the labeled arrows identifies hydrogen bonding in water?
A
B
C
D
6) A kinetics experiment is set up to collect the gas that is generated when a sample of chalk, consisting primarily of solid CaCO3 , is added to a solution of ethanoic acid, CH3COOH. The rate of reaction between CaCO3 and CH3COOH is determined by measuring the volume of gas generated at 25°C and 1 atm as a function of time. Which of the following experimental conditions is most likely to increase the rate of gas production?
Decreasing the volume of ethanoic acid solution used in the experiment
Decreasing the concentration of the ethanoic acid solution used in the experiment
Decreasing the temperature at which the experiment is performed
Decreasing the particle size of the CaCO3 by grinding it into a fine powder
2013 AP Chemistry化学模考MC选择题完整版答案免费下载
请持续关注,稍后更新
2013 AP Chemistry Sample Exam Multiple Choice Free Download
2013 AP 化学样卷选择题部分免费下载
此套Section I试卷共分计时1小时30分钟组成
共75题
仅限使用铅笔,无计算器
考试时会提供花常用的等式与常量
以及化学元素周期表
完整版真题资料可以底部二维码免费领取↓↓↓

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








