- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
2014 AP Chemistry化学真题系列之选择题免费下载
历年AP Calculus BC微积分BC系列
真题与答案下载

翰林国际教育全网首发
力争超快速发布最全资料
助你在升学路上一帆风顺
为你的未来保驾护航
2014 AP Chemistry Practice Exam Multiple Choice Free Download
2014 AP 化学模考选择题部分免费下载
此套Section I试卷共分计时1小时30分钟组成
共50题
仅限使用铅笔,无计算器
考试时会提供花常用的等式与常量
以及化学元素周期表
完整版下载链接见文末
部分真题预览:

1)According to the information in the table above, a 1.00g sample of which of the following contains the greatest mass of oxygen?
- Na2O
- MgO
- K2O
- CaO
2)Which of the following could be the identity of a white crystalline solid that exhibits the following properties?
- It melts at 320°C
- It does not conduct electricity as a solid.
- It conducts electricity in an aqueous solution.
- C6H12O6(s)
- NaOH(s)
- SiO2(s)
- Cu(s)
3)Which of the following correctly identifies which has the higher first-ionization energy, Cl or Ar and supplies the best justification?
- Cl, because of its higher electronegativity
- Cl, because of its higher electron affinity
- Ar, because of its completely filled valence shell
- Ar, because of its higher effective nuclear charge
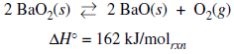
4)A sealed rigid vessel contains BaO2(s) in equilibrium with BaO(s) and O2(g) as represented by the equation above. Which of the following changes will increase the amount of BaO2(s) in the vessel?
- Removing a small amount of O2(g)
- Removing a small amount of BaO(s)
- Adding He(g) to the vessel
- Lowering the temperature
5) Which of the following best helps to explain why the value of Δ H° for the dissolving of CaF2(aq) in water is positive?
- CaF2(s) is insoluble in water.
- CaF2(s) dissolves in water to form CaF2(aq) particles.
- Ca2+ ions have very strong ion-ion interactions with F- ions in the crystal lattice.
- Ca2+ ions have very strong ion-dipole interactions with water molecules in the solution.
6)Under which of the following conditions of temperature and pressure will H2 gas be expected to behave most like an ideal gas?
- 50 K and 0.10 atm
- 50 K and 5.0 atm
- 500 K and 0.10 atm
- 500 K and 50 atm
完整版真题下载链接请注册或登录后查看
文件为PDF格式
推荐使用电脑下载
2014 AP Chemistry化学模考MC选择题完整版答案免费下载
请持续关注,稍后更新
翰林学员全站资料免费打包下载,专享高速下载通道。

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








