- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
2017 AP Chemistry化学真题系列之选择题免费下载
历年AP Calculus BC微积分BC系列
真题与答案下载

翰林国际教育全网首发
力争超快速发布最全资料
助你在升学路上一帆风顺
为你的未来保驾护航
2017 AP Chemistry Practice Exam Multiple Choice Free Download
2017 AP 化学模考选择题部分免费下载
此套Section I试卷计时1小时30分钟
共50题
仅限使用铅笔,无计算器
考试时会提供花常用的等式与常量
以及化学元素周期表
完整版下载链接见文末
部分真题预览:
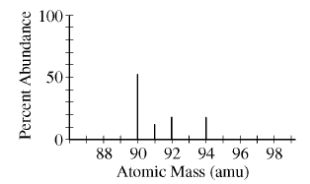
1)The mass spectrum of an average sample of a pure element is shown in the figure above. Which of the following is the identity of the element?
- Y
- Zr
- Nb
- Th
2)The ideal gas law best describes the properties of which of the following gases at 0°C and 1 atm?
- PH3
- HBr
- SO2
- N2
3)At 298 K and 1 atm, Br2 is a liquid with a high vapor pressure, and Cl2 is a gas. Those observations provide evidence that under the given conditions, the
- forces among Br2 molecules are stronger than those among Cl2 molecules
- forces among Cl2 molecules are stronger than the Cl−Cl bond
- Br−Br bond is stronger than the Cl−Cl bond
- Cl−Cl bond is stronger than the Br−Br bond
4) Which of the following has the bonds arranged in order of decreasing polarity?
- H−F > N−F > F−F
- H−I > H−Br > H−F
- O−N > O−S > O−Te
- Sb−I > Sb−Te > Sb−Cl
![]()
5) To determine the moles of Fe3+(aq) in a 100. mL sample of an unknown solution, excess KSCN(s) is added to convert all the Fe3+(aq) into the dark red species FeSCN2+(aq) , as represented by the equation above. The absorbance of FeSCN2+(aq) at different concentrations is shown in the graph below.
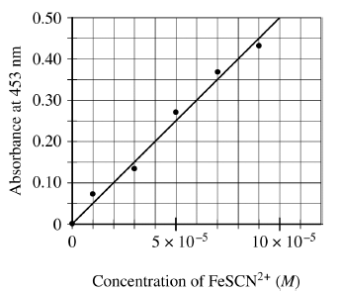
If the absorbance of the mixture is 0.20 at 453 nm, how many moles of Fe3+(aq) were present in the 100. mL sample? (Assume that any volume change due to adding the KSCN(s) is negligible.)
- 4 × 10−4 mol
- 3 × 10−4 mol
- 4 × 10−6 mol
- 3 × 10−6 mol
6)The first ionization energy of an element is the energy required to remove an electron from a gaseous atom of the element (i.e., X(g) → X+(g) + e− ). The values of the first ionization energies for the third-row elements are shown in the graph above. On the basis of the information given, which of the following reactions is exothermic?
- Cl(g) + Mg+(g) → Cl+(g) + Mg(g)
- Al(g) + Mg+(g) → Al+(g) + Mg(g)
- P(g) + Mg+(g) → P+(g) + Mg(g)
- S(g) + Mg+(g) → S+(g) + Mg(g)
完整版真题下载链接扫码免费领取

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









