- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE IGCSE Biology: 复习笔记:1.1.4 Diffusion
CIE IGCSE Biology: 复习笔记:1.1.4 Diffusion
Diffusion
- This is the process by which different gases or different liquids mix and is due to the random motion of their particles
- Diffusing particles move from an area of high concentration to an area of low concentration
- Eventually the concentration of particles is even as they spread out to occupy all of the available space
- Diffusion happens on its own and no energy input is required although it occurs faster at higher temperatures
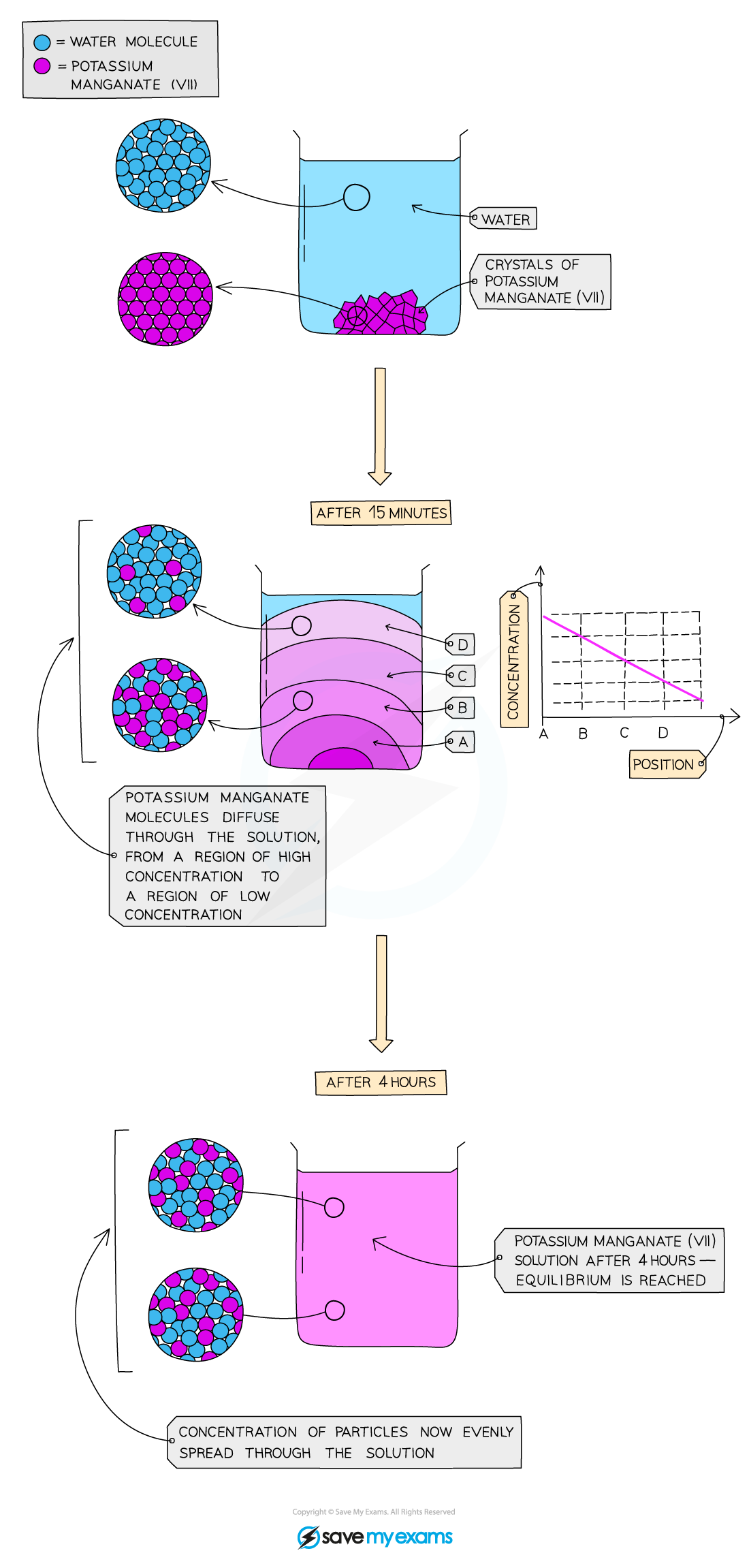
Diffusion of potassium manganate(VII), KMnO4 , in water. After a few hours the concentration of KMnO4 is the same throughout the solution
Diffusion & Molecular Mass
EXTENDED
- Diffusion occurs much faster in gases than in liquids as gaseous particles move much quicker than liquid particles
- At the same temperature, different gases do not diffuse at the same rate.
- This is due to the difference in their relative molecular masses
- Lighter gas particles can travel faster and hence further, therefore the lower its relative mass the faster a gas will diffuse
- This can be demonstrated in the reaction between ammonia, NH3, and hydrogen chloride gas, HCl, inside a long glass tube
- Where the two gases meet a white smoke of ammonium chloride, NH4Cl, is formed
- This does not occur in the middle of the tube as you might expect, but much closer to the end with the hydrogen chloride (Mr = 36.5) and the ammonia (Mr = 17) molecules are smaller and lighter
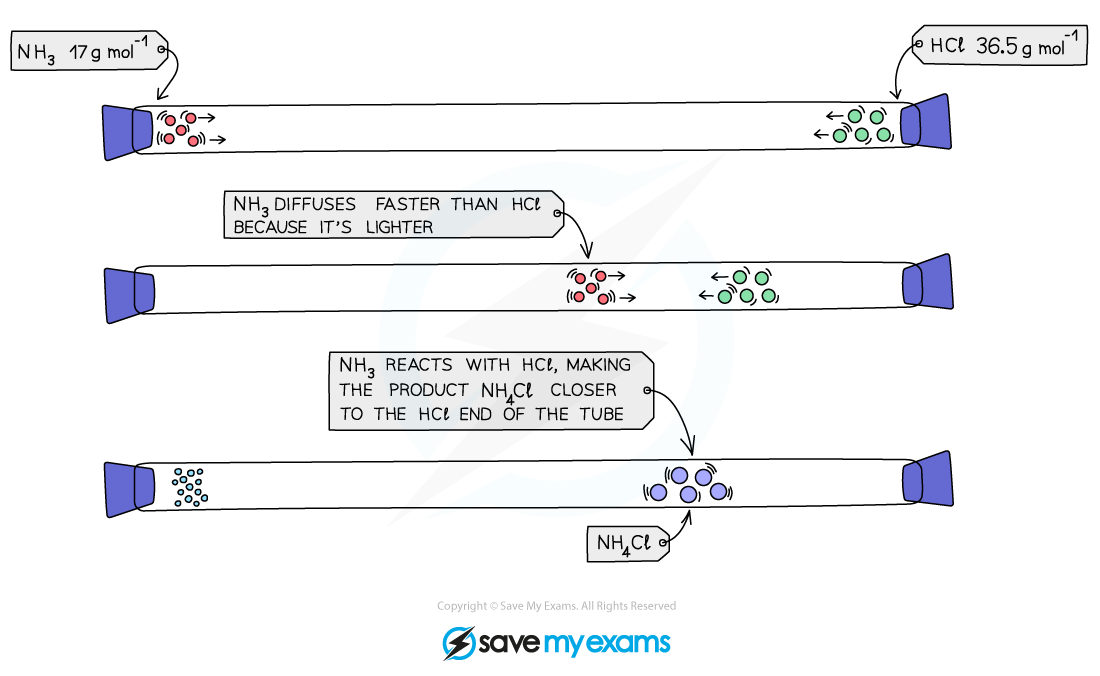 NH3 molecules have less mass than the HCl molecule, so diffuse faster, hence the product (a white smoke of NH4Cl) forms closer to the end where the HCl is
NH3 molecules have less mass than the HCl molecule, so diffuse faster, hence the product (a white smoke of NH4Cl) forms closer to the end where the HCl is
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









