- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
OCR A Level Biology:复习笔记2.4.4 Enzyme Activity: Temperature
Enzyme Activity: Temperature
- Enzymes have a specific optimum temperature
- This is the temperature at which they catalyse a reaction at the maximum rate
- Lower temperatures either prevent reactions from proceeding or slow them down because:
- Molecules move relatively slowly as they have less kinetic energy
- Less kinetic energy results in a lower frequency of successful collisions between substrate molecules and the active sites of the enzymes which leads to less frequent enzyme-substrate complex formation
- Substrates and enzymes also collide with less energy, making it less likely for bonds to be formed or broken (stopping the reaction from occurring)
- Higher temperatures cause reactions to speed up because:
- Molecules move more quickly as they have more kinetic energy
- Increased kinetic energy results in a higher frequency of successful collisions between substrate molecules and the active sites of the enzymes which leads to more frequent enzyme-substrate complex formation
- Substrates and enzymes also collide with more energy, making it more likely for bonds to be formed or broken (allowing the reaction to occur)
Denaturation
- If temperatures continue to increase past a certain point, the rate at which an enzyme catalyses a reaction drops sharply, as the enzyme begins to denature:
- The increased kinetic energy and vibration of the enzyme molecules puts a strain on them, eventually causing the weaker hydrogen and ionic bonds that hold the enzyme molecule in its precise shape to start to break
- The breaking of bonds causes the tertiary structure of the protein (i.e. the enzyme) to change
- The active site is permanently damaged and its shape is no longer complementary to the substrate, preventing the substrate from binding
- Denaturation has occurred if the substrate can no longer bind
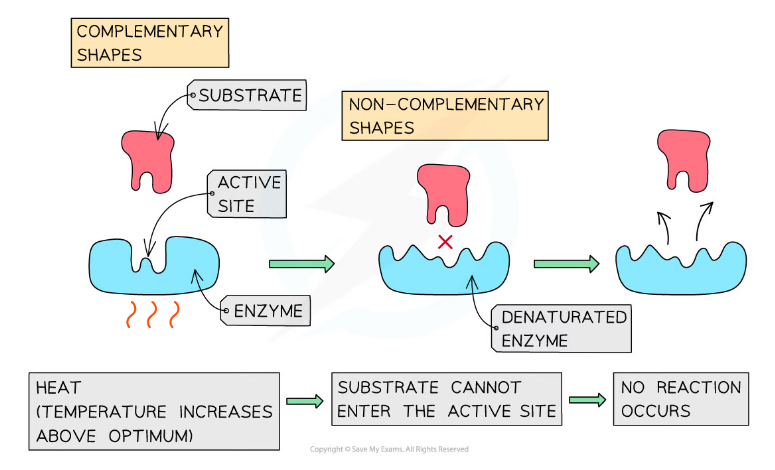
At high temperatures enzymes are denatured - the active site changes shape and is no longer complementary to the substrate. This change is irreversible.
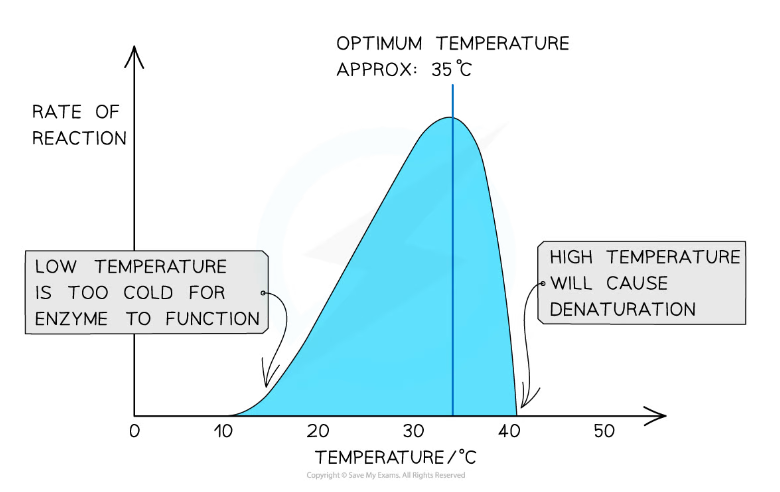
The effect of temperature on the rate of an enzyme-catalysed reaction
- The optimum temperature of an enzyme and the temperature at which an enzyme is denatured varies according to the habitat to which an organism is adapted
- Most enzymes present in living organisms denature at temperatures above 60 °C
- Very few human enzymes can function at temperatures above 50 °C
- Humans maintain a body temperature of about 37 °C and even temperatures exceeding 40 °C can cause the denaturation of some enzymes
- Some bacteria that live in thermal springs have enzymes that can withstand temperatures in excess of 80 °C
- These enzymes are thermostable
Temperature coefficient
- The temperature coefficient for a biological reaction is the ratio between the rates of that reaction at two different temperatures
- For most enzyme-catalysed reactions the rate of the reaction doubles for every 10 °C increase in temperature
- The temperature coefficient (Q) for a reaction that follows this pattern is: Q₁₀ = 2
- The temperature coefficient can be calculated using the following equation:
Temperature coefficient = (rate of reaction at (x + 10) °C) ÷ (rate of reaction at x °C)
Worked Example
The graph below shows the effect of temperature on an enzyme-catalysed reaction. Using the information in the graph, calculate the temperature coefficient for the reaction between 20 °C and 30 °C.
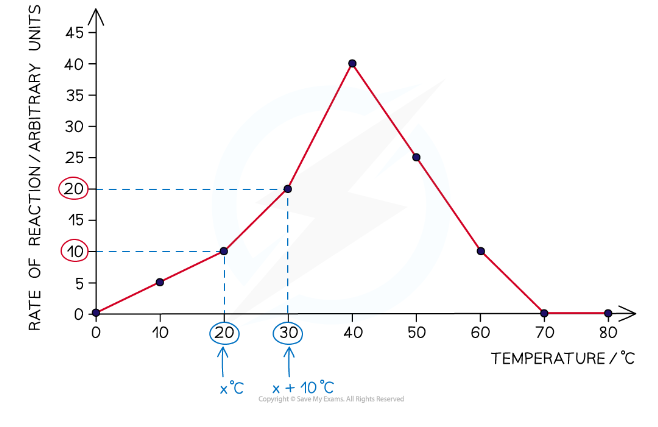
Step One: Using the graph, note the intercepts on the vertical axis at 20 °C and 30 °C
At 20 °C the rate of reaction is 10 arbitrary units and at 30 °C the rate of reaction is 20 arbitrary units
Step Two: Write out the equation and substitute in the known values
Temperature coefficient = (rate of reaction at (x + 10) °C) ÷ (rate of reaction at x °C)
Q₁₀ = rate of reaction at 30 °C ÷ rate of reaction at 20 °C
Q₁₀ = 20 ÷ 10
Step Three: Calculate the temperature coefficient
Q₁₀ = 2
(There is no unit for Q₁₀ as it is a ratio)
Exam Tip
When answering questions about reaction rates for enzyme-catalysed reactions, make sure to explain how the temperature affects the speed at which the molecules (enzymes and substrates) are moving (i.e. their kinetic energy) and how this, in turn, affects the number of successful collisions.A common mistake in exams is to say that enzymes are 'killed' at high temperatures. This is not biologically accurate and you would be marked down for this, as enzymes are protein molecules, not living organisms. Enzymes are denatured, not killed.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








