- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
OCR A Level Biology:复习笔记1.2.11 Biochemical Tests: Lipids
Biochemical Tests: Lipids
Identifying biological molecules
- Different qualitative reagents can be used to identfy the presence of biological molecules in samples
- Ethanol is used to identify lipids
- Biuret reagent is used to identify proteins
- Benedicts solution and iodine are used to identify carbohydrates
- Qualitative reagents simply determine whether or not a substance is present in a sample
- The quantity or concentration of the substance present is not determined
Practical: the emulsion test for lipids
- The emulsion test can be carried out quickly and easily in a lab to determine if a sample contains lipids
- Lipids are nonpolar molecules that do not dissolve in water but will dissolve in organic solvents such as ethanol
Apparatus
- Test tubes
- Test tube rack
- Ethanol
- Pipettes
- Food sample
- Mortar and pestle (if food sample is solid)
- Water
- Gloves
Method
- Add ethanol to the sample to be tested
- Shake to mix
- Add the mixture to a test tube of water
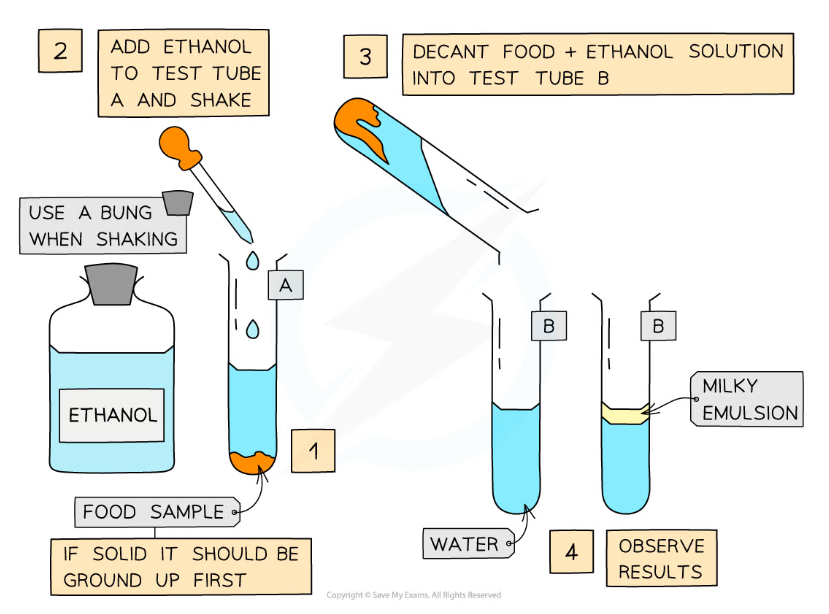
The Emulsion test for lipids forms a milky colour
Results
- If lipids are present, a milky emulsion will form (the solution appears ‘cloudy’); the more lipid present, the more obvious the milky colour of the solution
- If no lipid is present, the solution remains clear
Limitations
- This test is qualitative - it does not give a quantitative value as to how much lipid may be present in a sample
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









