- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.2.2 Combustion
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:4.2.2 Combustion
Fuels & Combustion
- The combustion of fossil fuels is the major source of atmospheric pollution
- Fossil fuels include: coal, oil, natural gas, oil shales and tar sands
- Non-renewable fossil fuels are obtained from crude oil by fractional distillation
- Petrol is used as a fuel in cars, kerosene is used to fuel aircraft and diesel oil is used as a fuel in some cars, trucks and heavy vehicles such as tanks and trains
- Coal is used in power stations and also steel production
- Natural gas consists mainly of methane, CH4
- There are finite amounts of fossil fuels and they all contribute to pollution and global warming
- All these fuels contain carbon, hydrogen and small quantities of sulfur
Combustion Products
- The burning of fossil fuels releases the gases carbon dioxide, carbon monoxide, oxides of nitrogen and oxides of sulfur
- In addition incomplete combustion of the fuels gives rise to unburned hydrocarbons and carbon particulates
Complete versus Incomplete Combustion
- A fuel is a substance which releases energy in an exothermic reaction
- When the fuel is a hydrocarbon then water and carbon dioxide are the products formed
- Hydrocarbon compounds undergo complete and incomplete combustion
- Complete combustion occurs when there is excess oxygen
- For example, the combustion equation for propane is:
C3H8 + 5O2 → 3CO2 + 4H2O
Incomplete Combustion
- Incomplete combustion occurs when there is insufficient oxygen to burn
- It occurs in some appliances such as boilers and stoves as well as in internal combustion engines
- The products of these reactions are unburnt fuel (soot), carbon monoxide and water
- Methane for example undergoes incomplete combustion in an oxygen-poor environment:
2CH4 + 3O2→ 2CO + 4H2O
CH4 + O2→ C + 2H2O
Exam Tip
You don't need to learn these equations, but you do need to be able to predict the products of combustion given the composition of the fuel and the conditions.
Dangers of Carbon Monoxide
- Carbon monoxide is a toxic and odourless gas which can cause dizziness, loss of consciousness and eventually death
- The CO binds well to haemoglobin which therefore cannot bind oxygen and carbon dioxide
- Oxygen is transported to organs
- Carbon dioxide is removed as waste material from organs
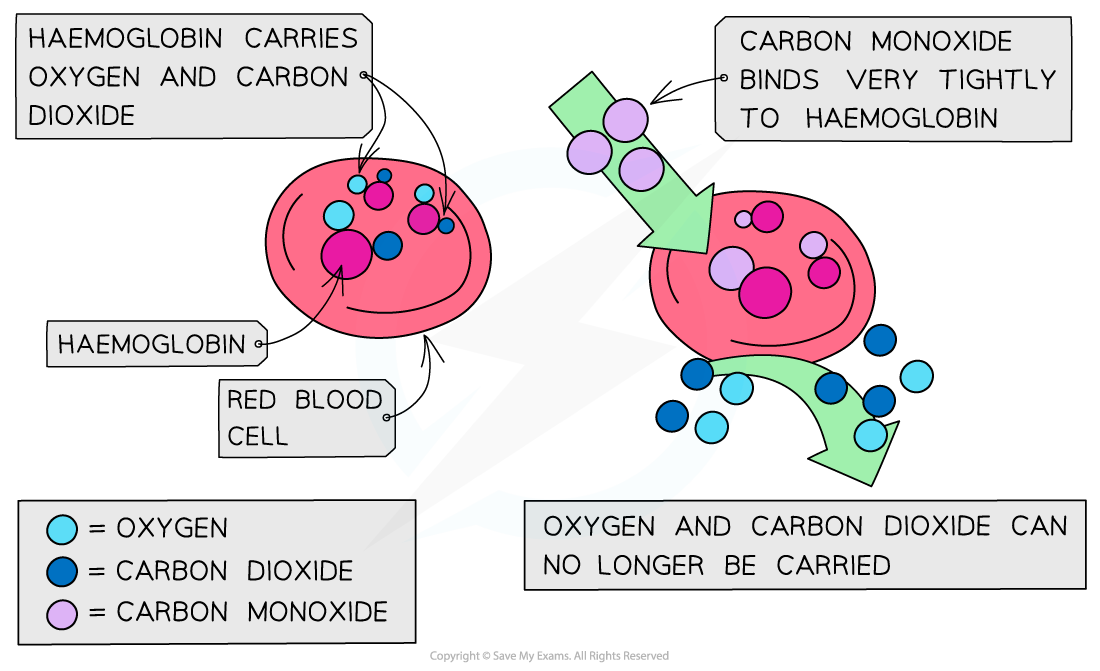
The high affinity of CO to haemoglobin prevents it from binding to O2 and CO2
Exam Tip
Though CO2 is not a toxic gas, it is still a pollutant causing global warming and climate change.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









