- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:3.2.3 Catalysts
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:3.2.3 Catalysts
Catalysts & Rates
- Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction
- The mass of a catalyst at the beginning and end of a reaction is the same and they do not form part of the equation
- An important industrial example is iron, which is used to catalyse the Haber Process for the production of ammonia
- Iron beads are used to increase the surface area available for catalysis
- Normally only small amounts of catalysts are needed to have an effect on a reaction
- Different processes require different types of catalysts but they all work on the same principle of providing an alternate route for the reaction to occur
- They do this by lowering the activation energy required, hence providing a reaction pathway requiring less energy
- Catalysis is a very important branch of chemistry in commercial terms as catalysts increase the rate of reaction (hence the production rate) and they reduce energy costs
- The transition metals are used widely as catalysts as they have variable oxidation states allowing them to readily donate and accept different numbers of electrons. This is key to their catalytic activity
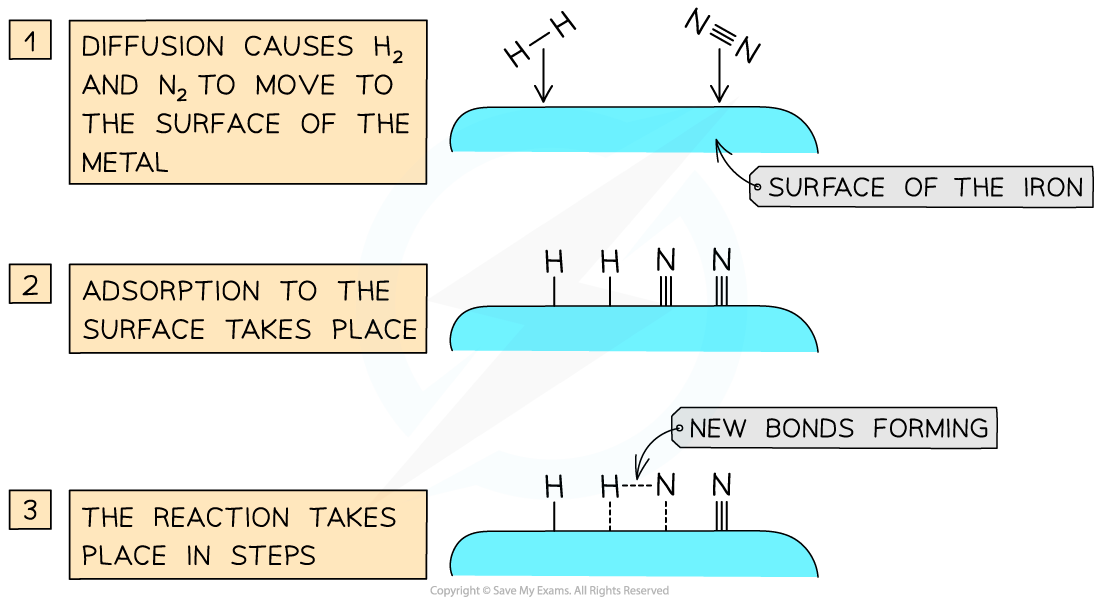
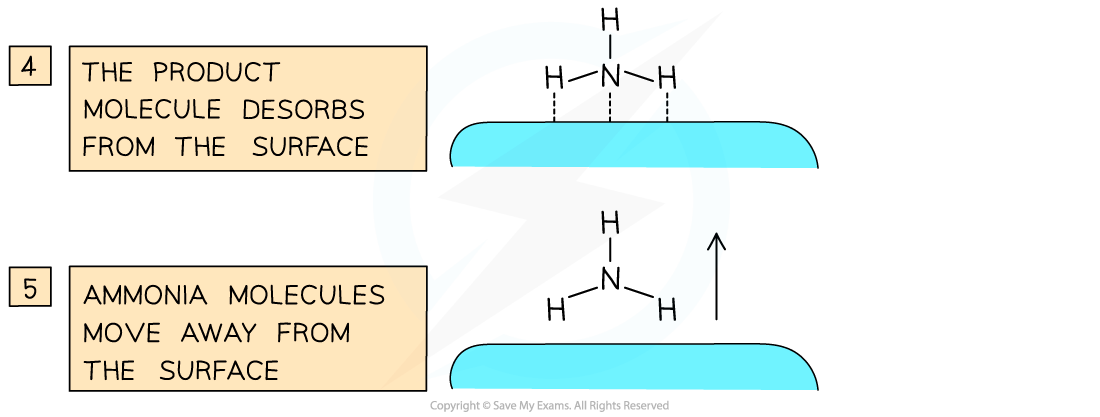
Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy
Exam Tip
Although catalysts are not part of the overall reaction, you may see them written over the arrow in reaction equations in the same way you can add reaction conditions above or below the arrow.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









