- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.6.6 Practical: Prepare Copper(II)Sulfate
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.6.6 Practical: Prepare Copper(II)Sulfate
Practical: Prepare Copper(II)Sulfate
Aim:
To prepare a pure, dry sample of hydrated copper(II) sulfate crystals
Materials:
- 1.0 mol / dm3 dilute sulfuric acid
- Copper(II) oxide
- Spatula & glass rod
- Measuring cylinder & 100 cm3 beaker
- Bunsen burner
- Tripod, gauze & heatproof mat
- Filter funnel & paper, conical flask
- Evaporating basin and dish.
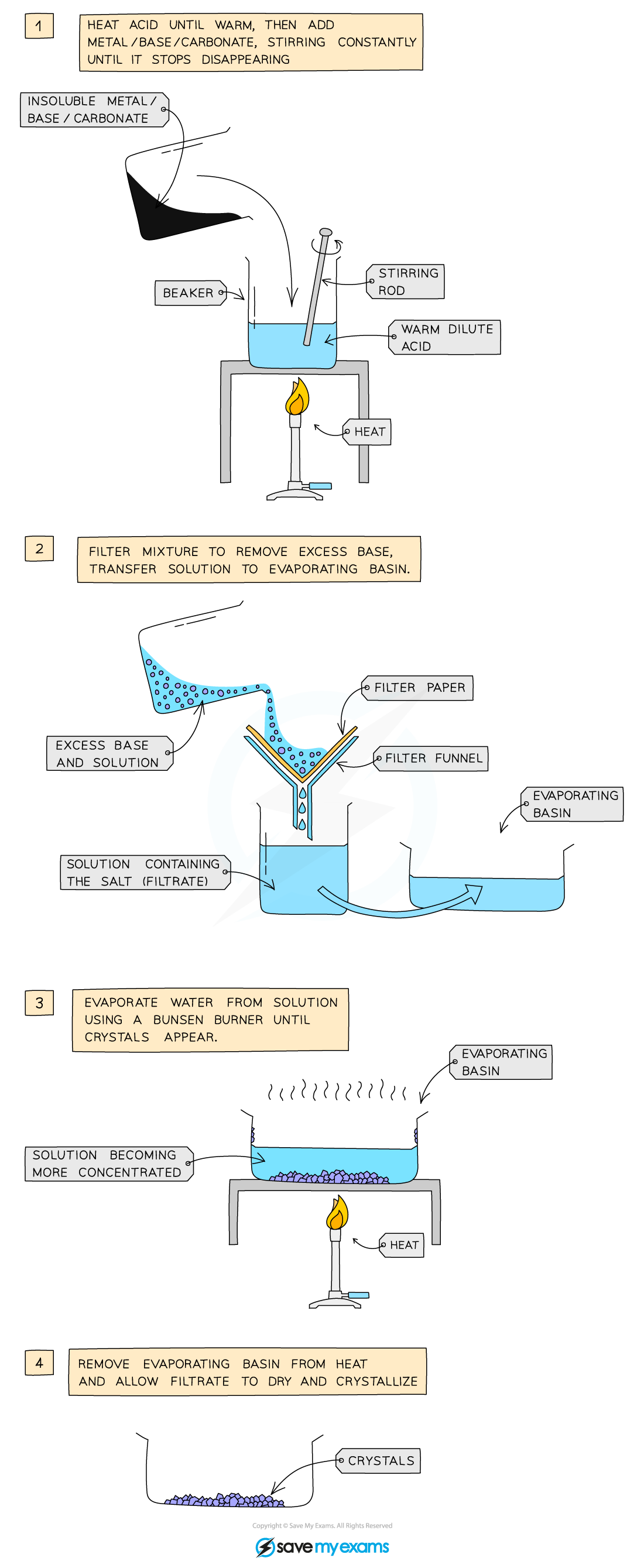
The preparation of copper(II) sulfate by the insoluble base method
Practical Tip:
The base is added in excess to use up all of the acid, which would become dangerously concentrated during the evaporation and crystallisation stages
Method:
- Add 50 cm3 dilute acid into a beaker and warm gently using a Bunsen burner
- Add the copper(II) oxide slowly to the hot dilute acid and stir until the base is in excess (i.e. until the base stops dissolving and a suspension of the base forms in the acid)
- Filter the mixture into an evaporating basin to remove the excess base
- Gently heat the solution in a water bath or with an electric heater to evaporate the water and to make the solution saturated
- Check the solution is saturated by dipping a cold glass rod into the solution and seeing if crystals form on the end
- Leave the filtrate in a warm place to dry and crystallise
- Decant excess solution and allow the crystals to dry
Results:
Hydrated copper(II) sulfate crystals should be bright blue and regularly shaped
Exam Tip
Make sure you learn the names of all the laboratory apparatus used in the preparation of salts.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








