- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.4.4 Rusting of Iron
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.4.4 Rusting of Iron
Rusting of Iron
- Rusting is a chemical reaction between iron, water and oxygen to form hydrated iron(III)oxide
- Oxygen and water must be present for rusting to occur
- Rusting is a redox process and it occurs faster in salty water since the presence of sodium chloride speeds up the reaction
Iron + Water + Oxygen → Hydrated Iron(III) Oxide
4Fe (s) + 3O2 (g) + xH2O (l) → 2Fe2O3.xH2O (s)
Investigating Rusting
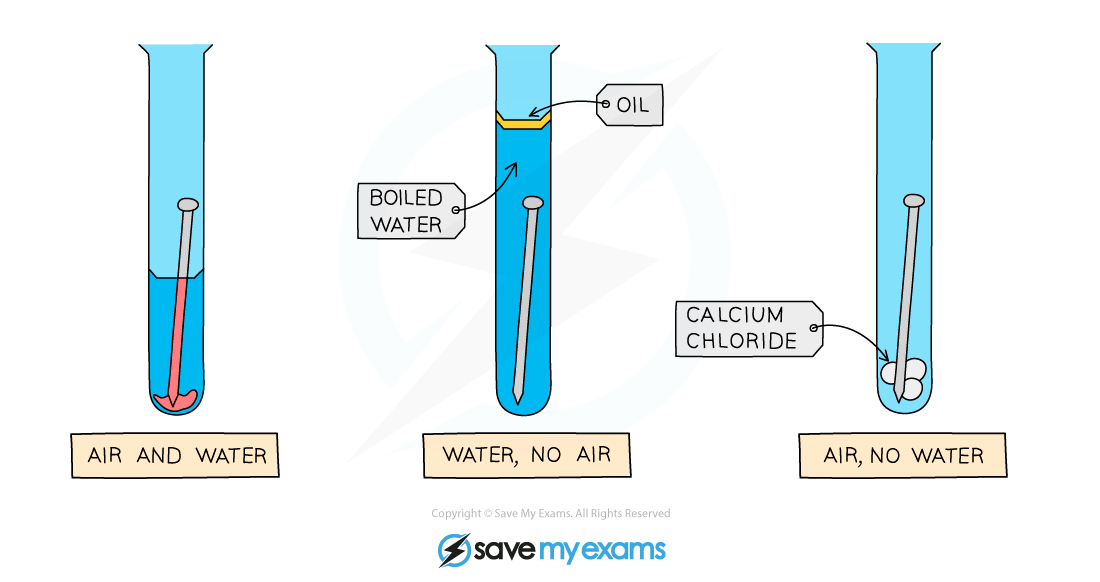 Diagram showing the requirements of oxygen and water for rust to occur: only the nail on the left rusts
Diagram showing the requirements of oxygen and water for rust to occur: only the nail on the left rusts
Method:
- Set up the apparatus as shown in the diagram
- The water in the second test tube is boiled to remove any dissolved oxygen
- The oil provides a barrier to prevent oxygen diffusing into the boiled water
- Calcium chloride is a drying agent in the third test tube
- Leave the apparatus for a few days to give it time to react
Results:
- The nail on the left rusts as it is in contact with both air (which contains oxygen) and water
- The nail in the middle does not rust as it is not in contact with air
- The nail on the right does not rust as it is not in contact with water (calcium chloride absorbs any water molecules present due to moisture)
- The results show that both air and water must be present for rusting to occur
Damage to Iron Structures
- Rust is a soft solid substance that flakes off the surface of iron easily, exposing fresh iron below which then undergoes rusting
- This means that over time all of the iron rusts and its structure becomes weakened
Preventing Iron Rusting
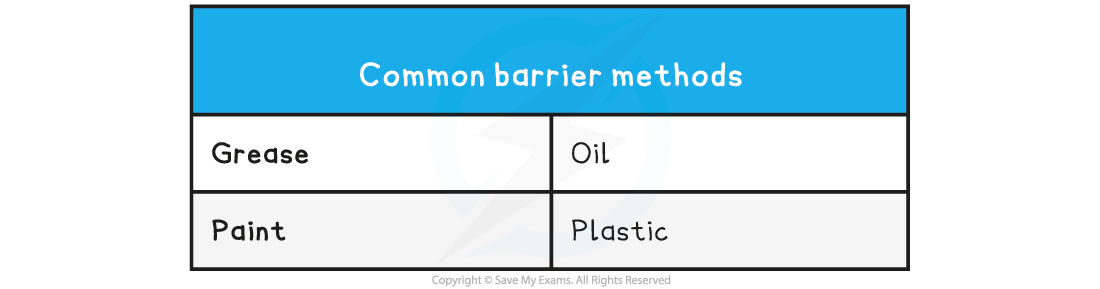
Barrier Methods
- Rust can be prevented by coating iron with barriers that prevent the iron from coming into contact with water and oxygen
- However, if the coatings are washed away or scratched, the iron is once again exposed to water and oxygen and will rust
Galvanising / Sacrificial protection
- Iron can be prevented from rusting making use of metals higher in reactivity than iron
- Galvanising is a process where the iron to be protected is coated with a layer of zinc
- ZnCO3 is formed when zinc reacts with oxygen and carbon dioxide in the air and protects the iron by the barrier method
- If the coating is damaged or scratched, the iron is still protected from rusting because zinc preferentially corrodes as it is higher up the reactivity series than iron
- Compared to iron it loses its electrons more readily:
Zn → Zn2+ + 2e–
- The iron stays protected as it accepts the electrons released by zinc, remaining in the reduced state and thus it does not undergo oxidation
- The electrons donated by the zinc react with hydrogen ions in the water producing hydrogen gas:
2H+ + 2e– → H2
- Zinc therefore reacts with oxygen and water and corrodes instead of the iron
Sacrificial Corrosion
- Sacrificial corrosion occurs when a more reactive metal is intentionally allowed to corrode
- An example of this occurs with ships’ hulls which sometimes have large blocks of magnesium or magnesium alloys attached
- The blocks slowly corrode and provide protection to the hull in the same way the zinc does by pushing electrons onto the iron which prevents it from being reduced to iron(III) ions
Exam Tip
Corrosion and rusting are not the same process. Corrosion is the general term used to describe the degradation of metal surfaces whereas rusting is the specific type of corrosion that happens to iron.
转载自savemyexams
在线登记
最新发布
翰林课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1