- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.3.2 Practical: Determine the % of Oxygen in Air
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.3.2 Practical: Determine the % of Oxygen in Air
ractical: Determine the Percentage of Oxygen in Air
Aim:
To determine the percentage of oxygen in air using the oxidation of iron
Diagram:
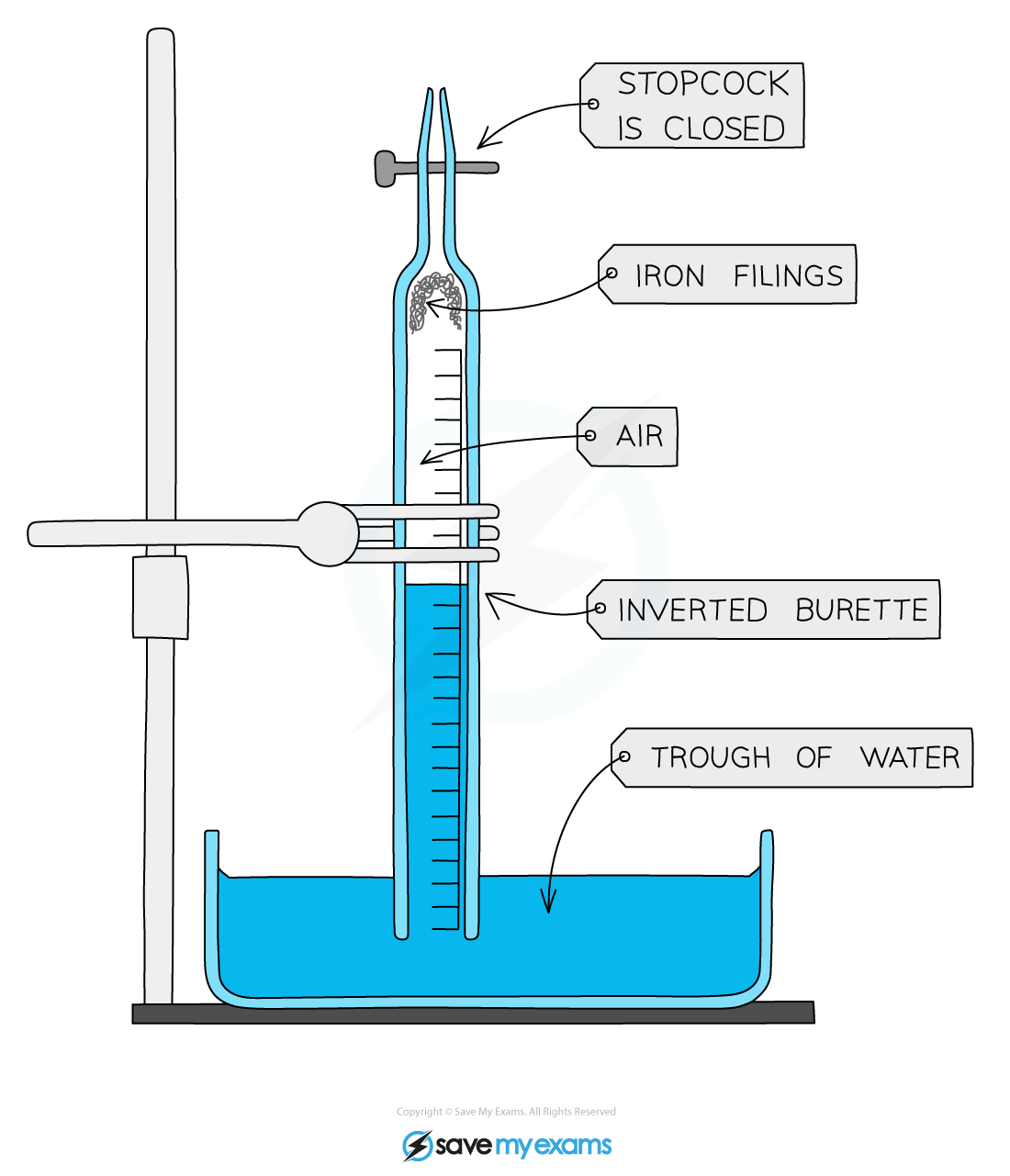
Apparatus to determine the percentage of oxygen in the air
Method:
- Firstly, you will need to measure the volume between the final mark on the scale and the tap (stopcock)
- Fill the burette with water up to lowest mark, 50.0 mL, and then let it drain into a small measuring cylinder
- Measure the volume of water
- Add a little water to moisten the inside of the burette
- Make sure the tap is closed and sprinkle some iron filings or push a piece of iron wool into the bottom of the burette
- Invert the burette into a trough of water and clamp the burette vertically
- Note and record the position of the water level
- After 3-4 days note the new position of the water level
Results:
Volume occupied between 50 mL & the tap = 3.8 mL
Initial water level = 2.6 mL
Final water level = 12.7 mL
Data Processing:
Initial volume of air = (50.0 + 3.8) - 2.6 = 51.2 mL
Final volume of air = 53.8 - 12.7 = 41.1 mL
Volume of oxygen = 51.2 - 41.1 = 10.1 mL
Percentage of oxygen = (10.1 ÷ 51.2) x 100 = 19.7%
Conclusion:The oxygen takes up approximately 20% of the air
转载自savemyexams

在线登记
最新发布
翰林课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1








