- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.2.1 Group 7 (Halogens)
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:2.2.1 Group 7 (Halogens)
Physical Properties
- The elements in group 7 are known as the halogens
- These are fluorine, chlorine, bromine, iodine and astatine
- These elements are non-metals that are poisonous
- All halogens have similar reactions as they each have seven electrons in their outermost shell
- Halogens are diatomic, meaning they form molecules made of pairs of atoms sharing electrons (forming a single covalent bond between the two halogen atoms)
Trends in Physical Properties
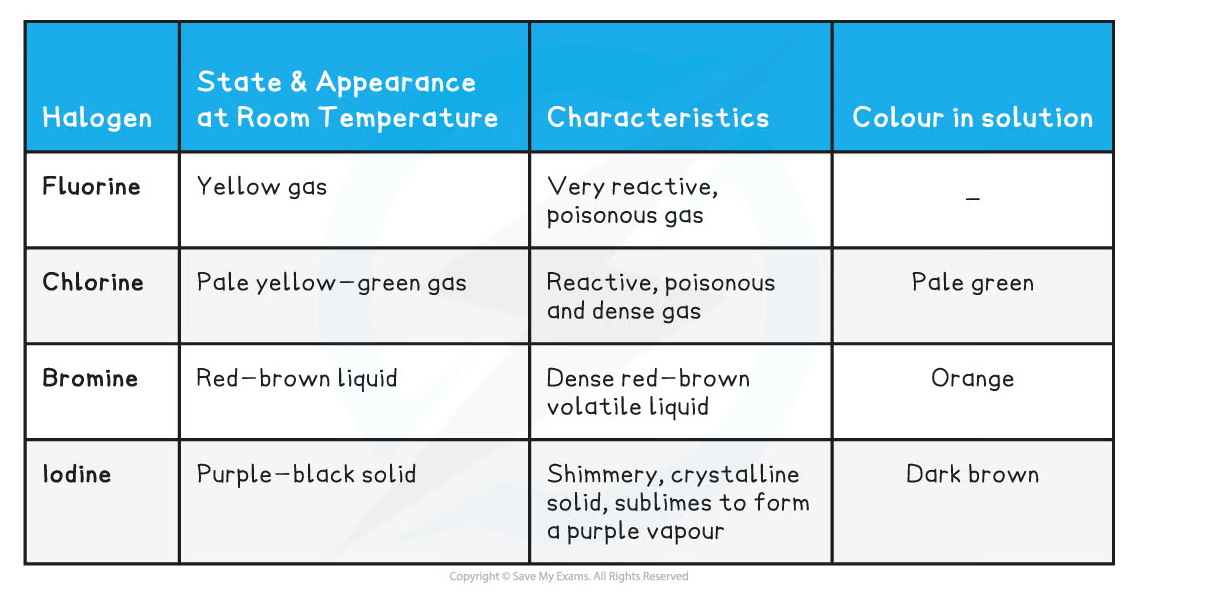
- At room temperature, the halogens exist in different states and colours, with different characteristics
The Appearance, Characteristics and Colour in Solution of the Halogens
- The melting and boiling points of the halogens increase as you go down the group
- This is due to increasing intermolecular forces as the atoms become larger, so more energy is required to overcome these forces
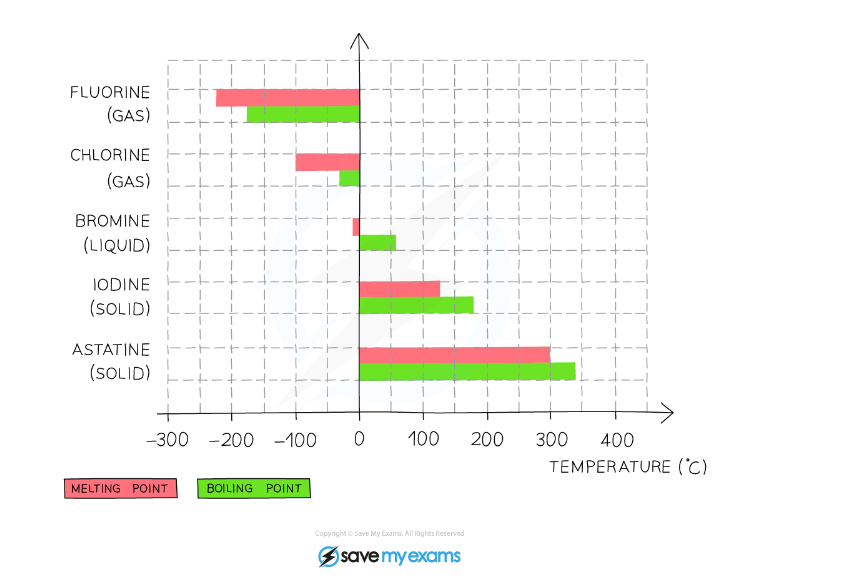
This graph shows the melting and boiling points of the group 7 halogens
- At room temperature (20 °C), the physical state of the halogens changes as you go down the group
- Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid
- The colours of the halogens also change as you descend the group - they become darker
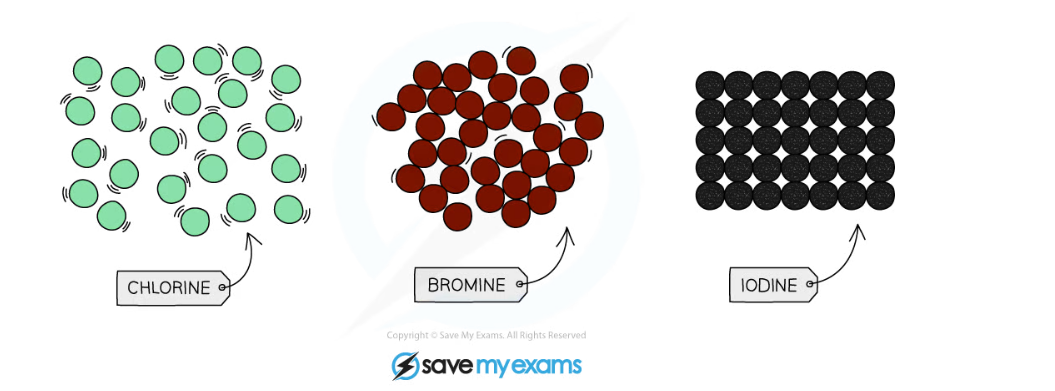
The physical states and colours of chlorine, bromine and iodine at room temperature
Exam Tip
Exam questions on this topic occur often so make sure you know and can explain the trends of the group 7 elements in detail, using their electron configurations.
Predicting Properties in Group 7
- Chlorine, bromine and iodine react with metals and non-metals to form compounds
Metal Halides
- The halogens react with some metals to form ionic compounds which are metal halide salts
- The halide ion carries a -1 charge so the ionic compound formed will have different numbers of halogen atoms, depending on the valency of the metal
- E.g., sodium is a group 1 metal:
- 2 Na + Cl2 → 2 NaCl
- Calcium is a group 2 metal:
- Ca + Br2 → CaBr2
- The halogens decrease in reactivity moving down the group, but they still form halide salts with some metals including iron
- The rate of reaction is slower for halogens which are further down the group such as bromine and iodine
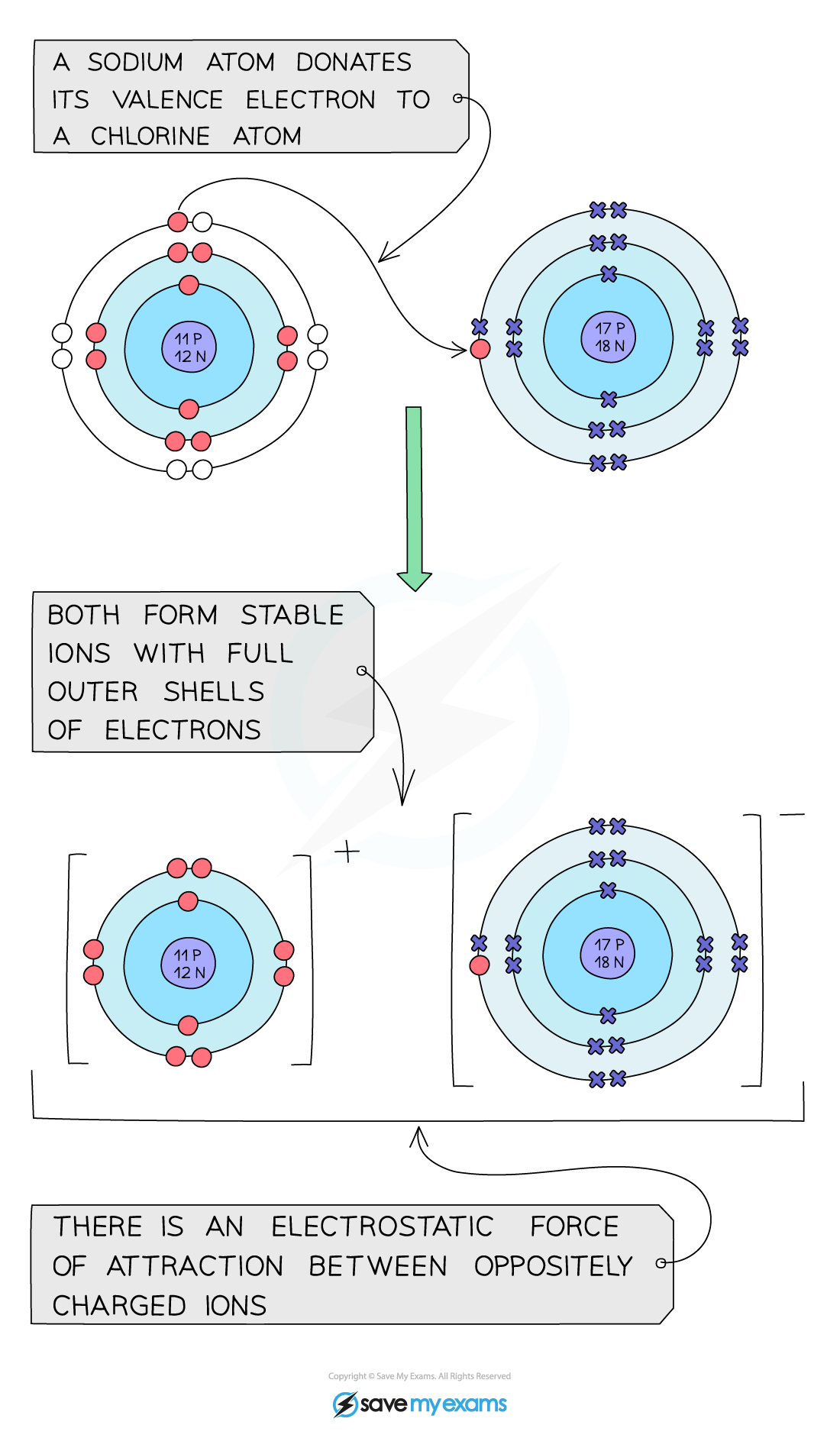
Sodium donates its single outer electron to a chlorine atom and an ionic bond is formed between the positive sodium ion and the negative chloride ion
Non-metal Halides
- The halogens react with non-metals to form simple molecular covalent structures
- For example, the halogens react with hydrogen to form hydrogen halides (e.g., hydrogen chloride)
- Reactivity decreases down the group, so iodine reacts less vigorously with hydrogen than chlorine (which requires light or a high temperature to react with hydrogen)
- Fluorine is the most reactive (reacting with hydrogen at low temperatures in the absence of light)
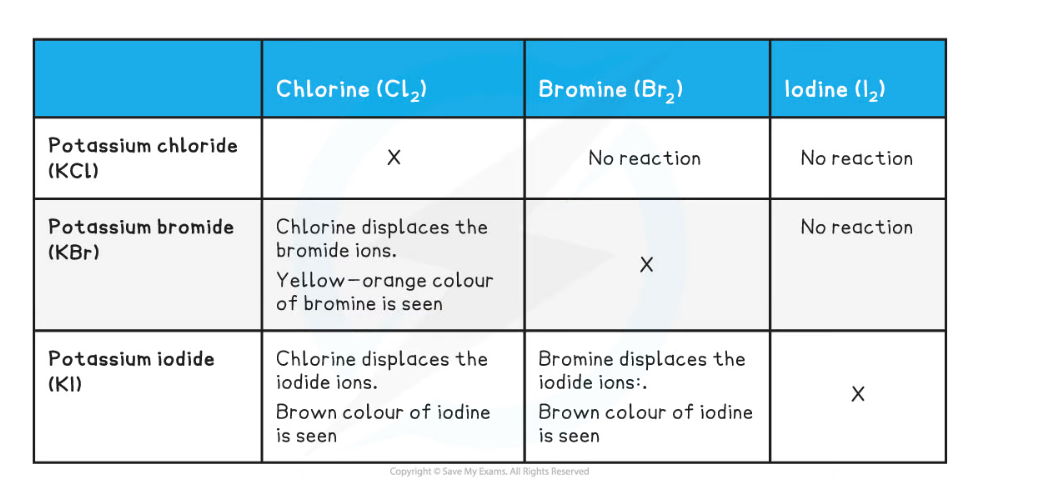
Displacement Reactions
- A halogen displacement reaction occurs when a more reactive halogen displaces a less reactive halogen from an aqueous solution of its halide
- The reactivity of group 7 elements decreases as you move down the group
- You only need to learn the displacement reactions with chlorine, bromine and iodine
- Chlorine is the most reactive and iodine is the least reactive
Chlorine with Bromides & Iodides
- If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs:
- The solution becomes orange as bromine is formed or
- The solution becomes brown as iodine is formed
- Chlorine is above bromine and iodine in group 7 so it is more reactive
- Chlorine will displace bromine or iodine from an aqueous solution of the metal halide:
Cl2 + 2KBr → 2KCl + Br2
chlorine + potassium bromide → potassium chloride + bromine
Cl2 + 2KI → 2KCl + I2
chlorine + potassium iodide → potassium chloride + iodine
Bromine with Iodides
- Bromine is above iodine in group 7 so it is more reactive
- Bromine will displace iodine from an aqueous solution of the metal iodide
bromine + potassium iodide → potassium bromide + iodine
Br2 + 2KI → 2KBr + I2
- This table shows a summary of the displacement reactions of the halogens: chlorine, bromine and iodine
Exam Tip
Displacement reactions are sometimes known as single replacement reactions.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









