- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.7.1 Formation of Covalent Bonds
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.7.1 Formation of Covalent Bonds
Formation of Covalent Bonds
- Non-metal atoms can share electrons with other non-metal atoms to obtain a full outer shell of electrons
- When atoms share pairs of electrons, they form covalent bonds
- Covalent bonds between atoms are very strong
- When two or more atoms are chemically bonded together, they form ‘molecules’
- Covalently bonded substances may consist of small molecules or giant molecules
- Weak intermolecular forces exist between individual molecules
- E.g. Each liquid water molecule consists of two hydrogen atoms covalently bonded to an oxygen atom, and in between two individual water molecules there are weak intermolecular forces
- Shared electrons are called bonding electrons and occur in pairs
- Electrons on the outer shell which are not involved in the covalent bond(s) are called non-bonding electrons
- Simple covalent molecules do not conduct electricity as they do not contain free electrons

Diagram showing covalent bonding in a molecule of chlorine (Cl2)
Exam Tip
A key difference between covalent bonds and ionic bonds is that in covalent bonds the electrons are shared between the atoms, they are not transferred (donated or gained) and no ions are formed.
Electrostatic Attractions
- There is a strong electrostatic attraction between the shared pair of electrons and the nuclei of the atoms involved, since the electrons are negatively charged and the nuclei are positively charged
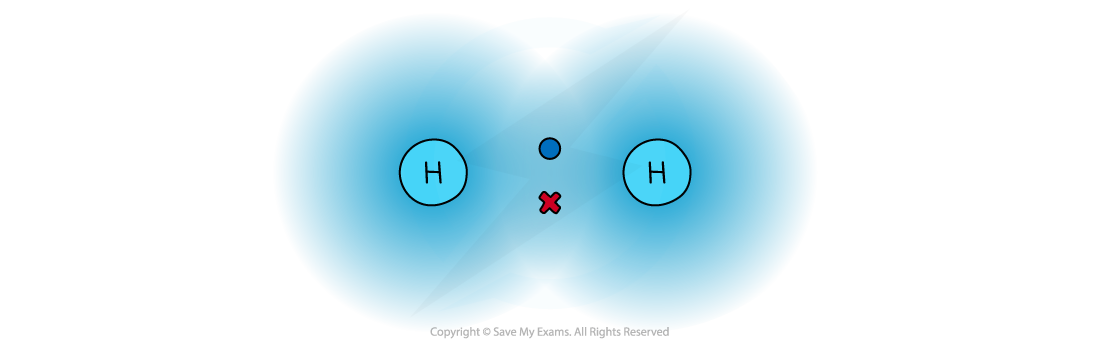
The attraction between the shared pair of electrons and the nuclei of the atoms involved in a covalent bond
- In a normal covalent bond, each atom provide one of the electrons in the bond. A covalent bond is represented by a short straight line between the two atoms, H-H
- Covalent bonds should not be regarded as shared electron pairs in a fixed position; the electrons are in a state of constant motion and are best regarded as charge clouds
- Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
- This makes each atom more stable
转载自savemyexams

在线登记
最新发布
翰林课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1








