- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds: Dot & Cross Diagrams
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds: Dot & Cross Diagrams
Ionic Bonds: Dot & Cross Diagrams
Deducing Dot & Cross Diagrams for Ionic Compounds
- Sodium is a group 1 metal so will lose one outer electron to another atom to gain a full outer shell of electrons
- A positive sodium ion with the charge 1+ is formed
- Chlorine is a group 7 non-metal so will need to gain an electron to have a full outer shell of electrons
- One electron will be transferred from the outer shell of the sodium atom to the outer shell of the chlorine atom
- A chlorine atom will gain an electron to form a negatively charged chloride ion with a charge of 1-
Formula of ionic compound: NaCl
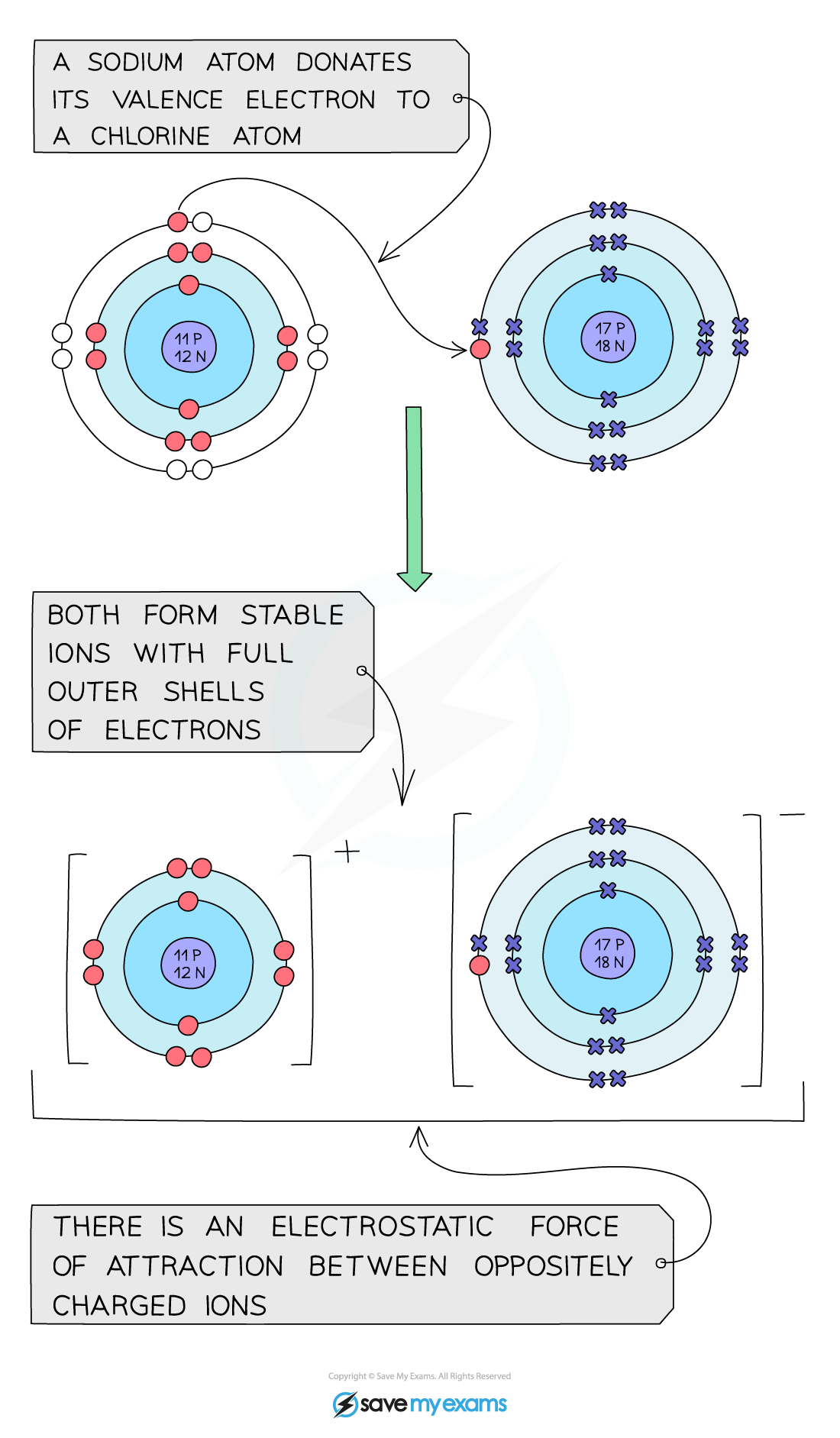 Sodium chloride ionic bonding
Sodium chloride ionic bonding
Exam Tip
For exam purposes you need only show the outer electrons in dot & cross diagrams.You should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7.
Magnesium Oxide Dot & Cross Diagram
- Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons
- A positive ion with the charge 2+ is formed
- Oxygen is a group 6 non-metal so will need to gain two electrons to have a full outer shell of electrons
- Two electrons will be transferred from the outer shell of the magnesium atom to the outer shell of the oxygen atom
- Oxygen atom will gain two electrons to form a negative ion with charge 2-
Formula of ionic compound: MgO
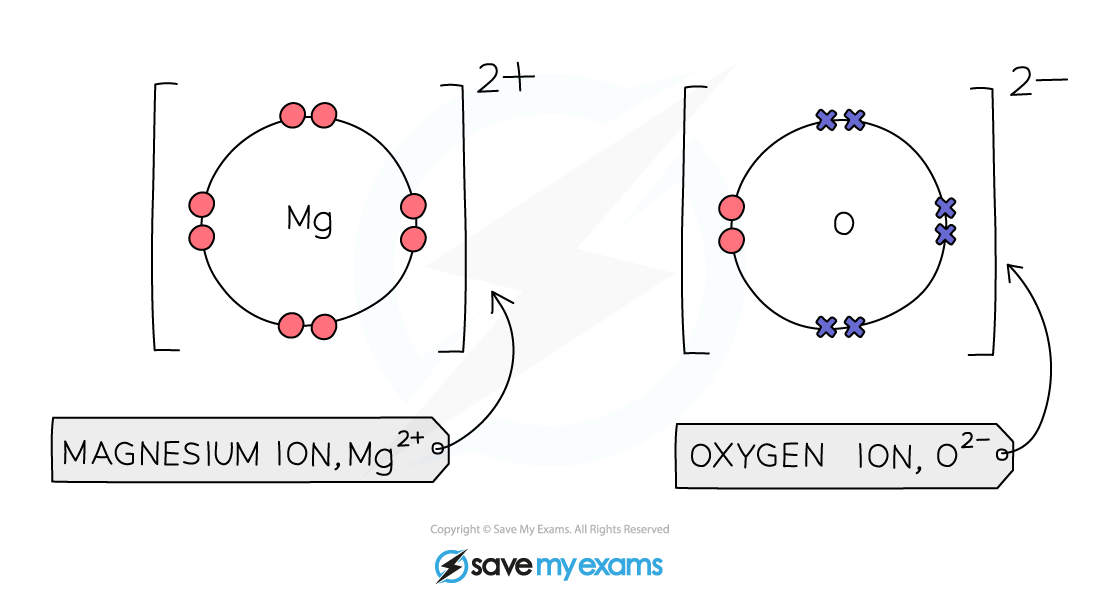
Diagram showing the dot-and-cross diagram of magnesium oxide
Exam Tip
When writing about ions, we use the notation 1-, 2+ etc. to describe the charge of the ion, with the number first followed by the sign (+/-). It is incorrect to write them the other way around as this refers to the oxidation state, not the charge.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








