- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.6 Experiment: Finding Formulae of Compounds
EDEXCEL IGCSE CHEMISTRY: DOUBLE SCIENCE 复习笔记:1.5.6 Experiment: Finding Formulae of Compounds
Formulae of Simple Compounds by Experiment
Making Careful Quantitative Measurements
- The formulae of simple compounds can be found by careful experimentation and accurate measurements of mass changes
- The principle is to use mass measurements before and after reaction and then convert masses into moles
- Using the moles of reactants and products it is possible to deduce molar ratios and hence an empirical formula
- Experiments which are easier to do using this process involve gases being lost or gained
- In this example a hydrated salt is heated to drive off the water as water vapour
The Formula of a Hydrated Salt
Aim:
To determine the formula of hydrated copper sulfate, CuSO4. xH2ODiagram:
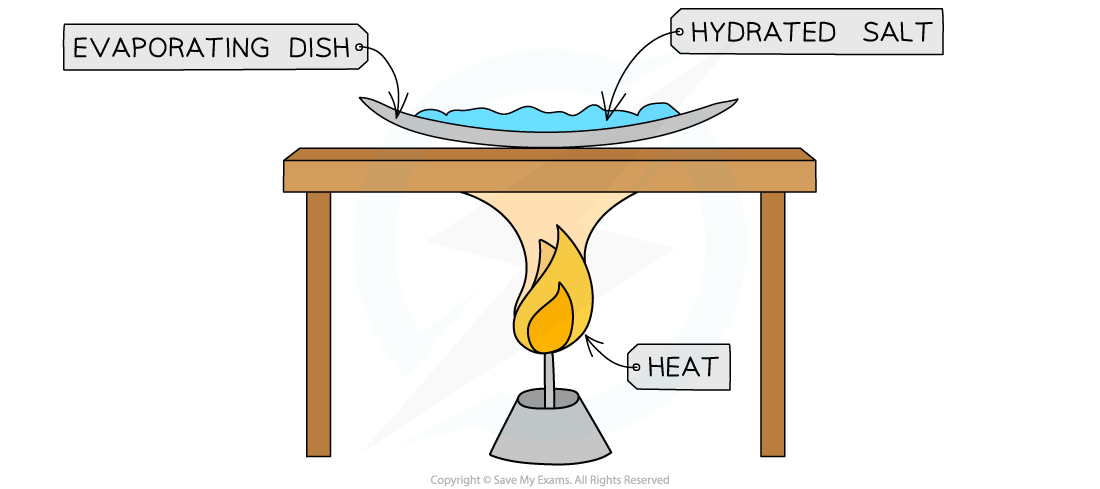
Heating a hydrated salt to remove the water of crystallisation
- Measure mass of evaporating dish
- Add a known mass of hydrated salt
- Heat over a Bunsen burner, gently stirring, until the blue salt turns completely white, indicating that all the water has been lost
- Record the mass of the evaporating dish and contents
Practical tip:
Avoid overheating the salt as it could decompose and give you a larger mass change
Mass of the white anhydrous salt:
Measure mass of white anhydrous salt remainingMass of water:Subtract mass of the white anhydrous salt remaining from the mass of known hydrated salt
Step 1 – Divide the mass of the copper sulfate and the water by their respective molar masses
Step 2 – Simplify the ratio of water to copper sulfate
anhydrous salt water
Mass a b
Moles a / Mr b / Mr
= y = x
Ratio 1 : x
Step 3 – Represent the ratio in the form ‘salt.xH2O’
Exam Tip
It is unlikely that you will get a whole number for the number of moles of water in the ratio, so you will need to round up or down to the nearest whole number.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









