Pure Substance vs Mixture
- In everyday language we use the word pure to describe when something is natural or clean and to which nothing else has been added
- In chemistry a pure substance may consist of a single element or compound which contains no other substances
- For example a beaker of a sample of pure water contains only H2O molecules and nothing else
- If salt were added to the beaker then a mixture is produced
- A mixture consists of two or more elements or compounds that are physically mixed together, they are not chemically combined
- The chemical properties of the substances in a mixture remain unchanged
- Substances in mixtures can be separated by physical means
- Air for example is a mixture of nitrogen, oxygen and some other gases such as carbon dioxide and argon

Diagram showing how to represent elements, compounds and mixtures using particle diagrams
Distinguishing Purity
- Pure substances melt and boil at specific and sharp temperatures e.g. pure water has a boiling point of 100 °C and a melting point of 0 °C
- Mixtures have a range of melting and boiling points as they consist of different substances that tend to lower the melting point and broaden the melting point range
- Melting and boiling points data can therefore be used to distinguish pure substances from mixtures
- Melting point analysis is routinely used to assess the purity of drugs
- This is done using a melting point apparatus which allows you to slowly heat up a small amount of the sample, making it easier to observe the exact melting point
- This is then compared to data tables
- The closer the measured value is to the actual melting or boiling point then the purer the sample is
Cooling Curves
- The influence of impurities can be more clearly seen on a heating / cooling curve
- If the temperature of a liquid is measured as it cools and freezes the data can be used to produce a graph
- The following graph shows the cooling curve for a sample of a compound
- The horizontal part of the graph shows that the compound has a sharp melting point, so the compound is pure
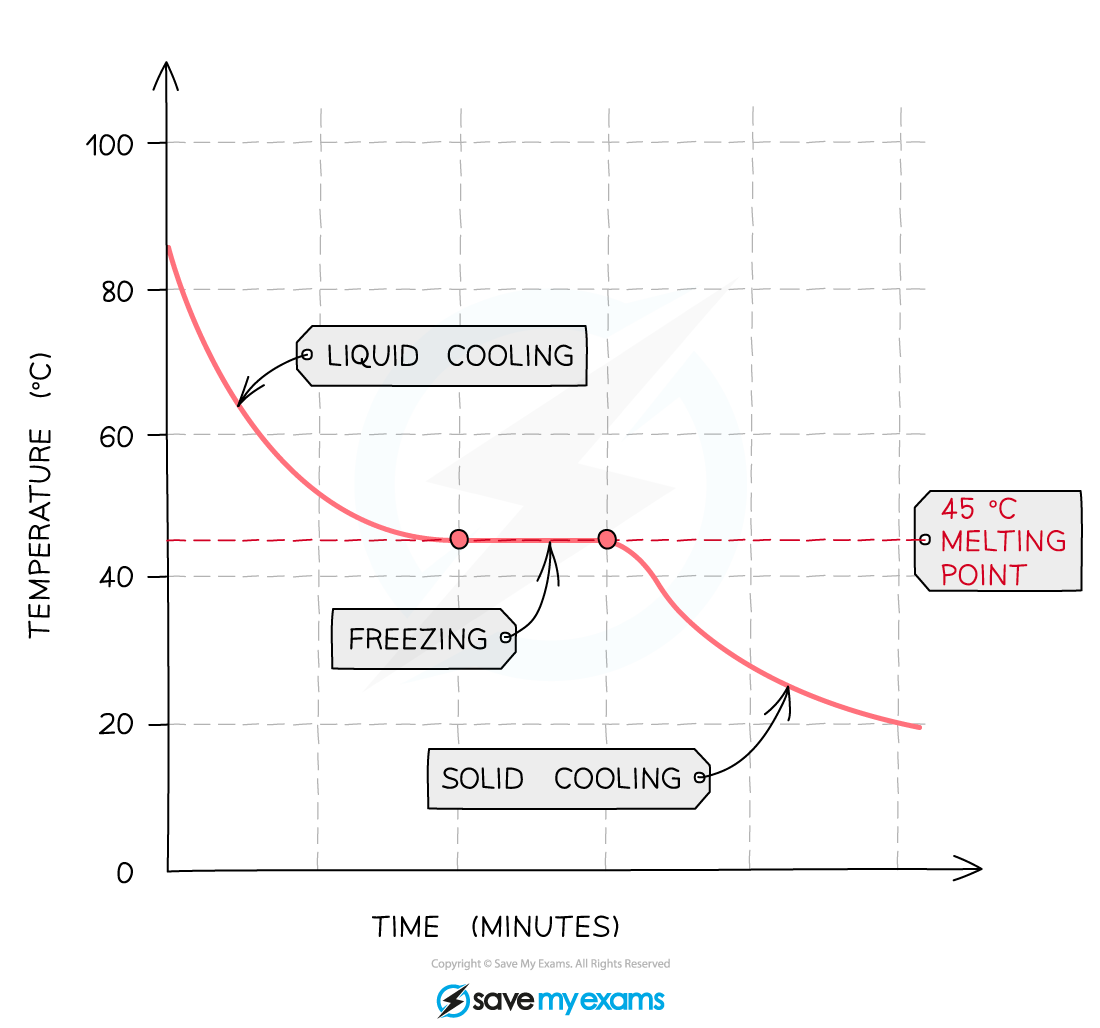
Cooling curve for a pure substance
- An impure sample of the compound would produce a gradual decrease in temperature as it freezes as shown in the graph below
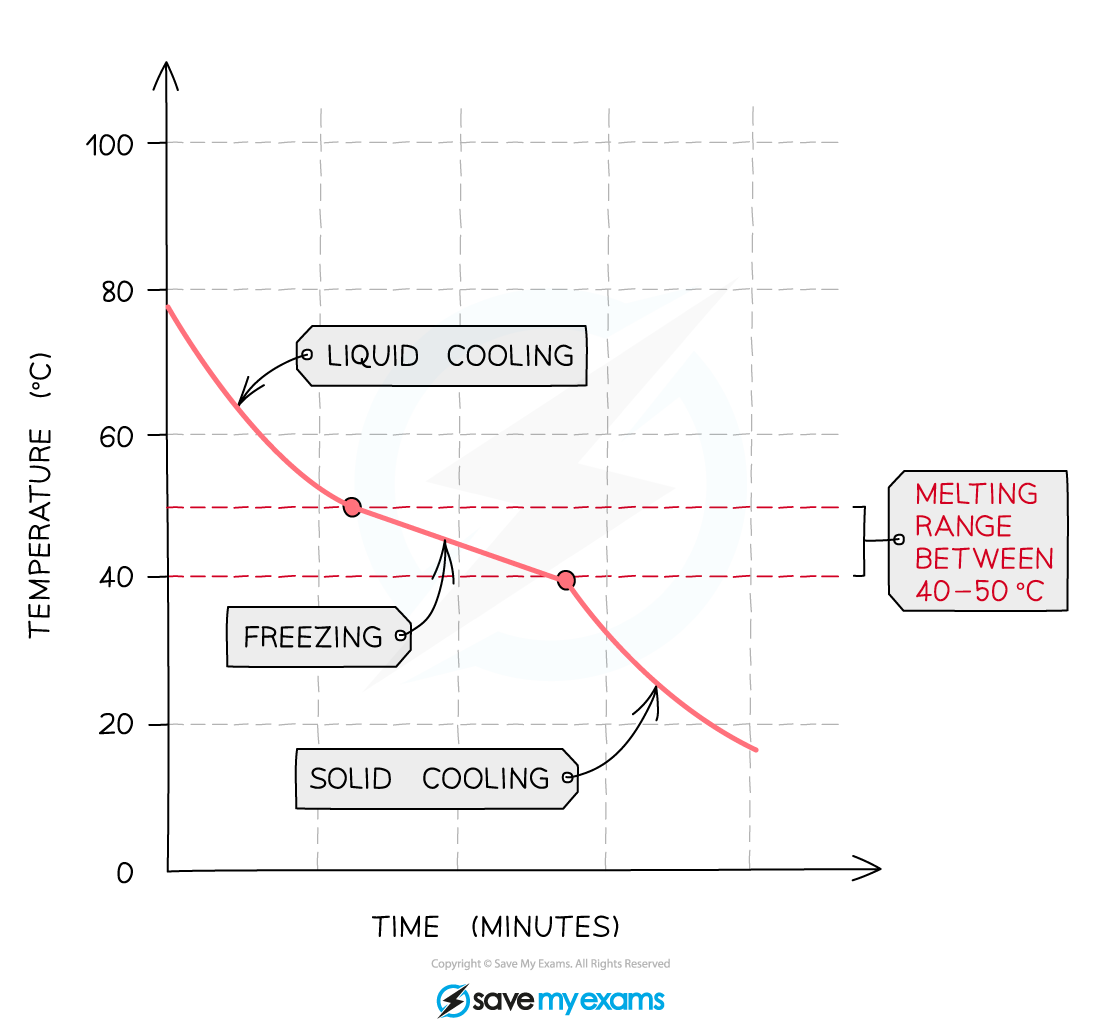
Cooling curve for an impure substance
转载自savemyexams










