- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A (SNAB) A Level Biology:复习笔记3.4.2 Stem Cells in Medicine
Stem Cells in Medicine
- As stem cells have the ability to differentiate into other specialised cell types, they are very valuable in medical research and treatments as they have the potential to replace damaged tissues and cells (that result from certain diseases)
- For example, many stem cell therapies already exist, including the treatment for leukaemia (bone marrow cancer), in which the existing stem cells in the bone marrow are killed, before being replaced using a bone marrow stem cell transplant, which will eventually replace all the bone marrow cells
- Other stem cell therapies are being researched, including therapies that replace damaged nerve tissue to treat spinal cord injuries and therapies that replace damaged heart tissue to treat heart disease and tissue damage caused by heart attacks
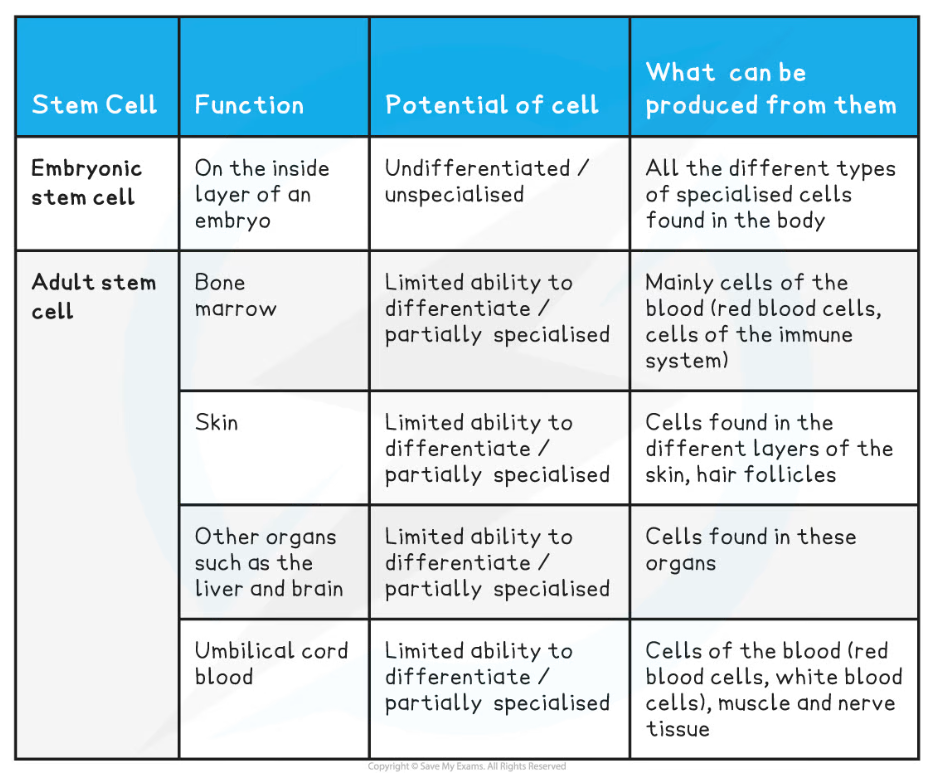
- There are two sources of human stem cells for use in medicine and research:
- Embryonic stem cells
- Adult stem cells
Use of embryonic stem cells
- Due to their ability to differentiate into almost any cell type, embryonic stem cells have huge potential in the therapeutic treatment of many diseases
- For many countries, such as the USA and some countries within the EU, the use of embryonic stem cells is banned, even for research
- In other countries, such as the UK, the use of embryonic stem cells is allowed for research but is very tightly regulated
- Embryonic stem cells can be one of two potencies:
- Totipotent if taken in the first 3-4 days after fertilisation
- Pluripotent if taken on day 5
- The embryos used for research are often the waste (fertilised) embryos from in vitro fertilisation (IVF) treatment
- This means these embryos have the potential to develop into human beings
- This is why many people have ethical objections to using them in research or medicine
Use of adult stem cells
- Adult stem cells can divide (by mitosis) an unlimited number of times but they are only able to produce a limited range of cell types
- A small number of adult stem cells are found in certain tissues within the body such as:
- Bone marrow - used to produce different types of blood cell
- Brain - used to produce different types of neural and glial cells
- These small numbers of stem cells remain to produce new cells for the essential processes of growth, cell replacement and tissue repair
- Research is being carried out on stem cell therapy, which is the introduction of adult stem cells into damaged tissue to treat diseases (e.g. leukaemia) and injuries (e.g. skin burns)
- The use of adult stem cells is less controversial than embryonic stem cells because the donor is able to give permission
- For example, many people donate bone marrow to help treat leukaemia patients
- However, if adult stem cells are being donated from one person to another they need to be a close match in terms of blood type and other body antigens
- Otherwise, there is a chance that the cells used are rejected by the patient's immune system (the cells in the stem cell transplant are recognised as being foreign and are attacked by the patient's immune system)
- Ideally, the patient's own adult stem cells are used to treat them, as there is a much lower chance of rejection
Sources of Human Stem Cells Table
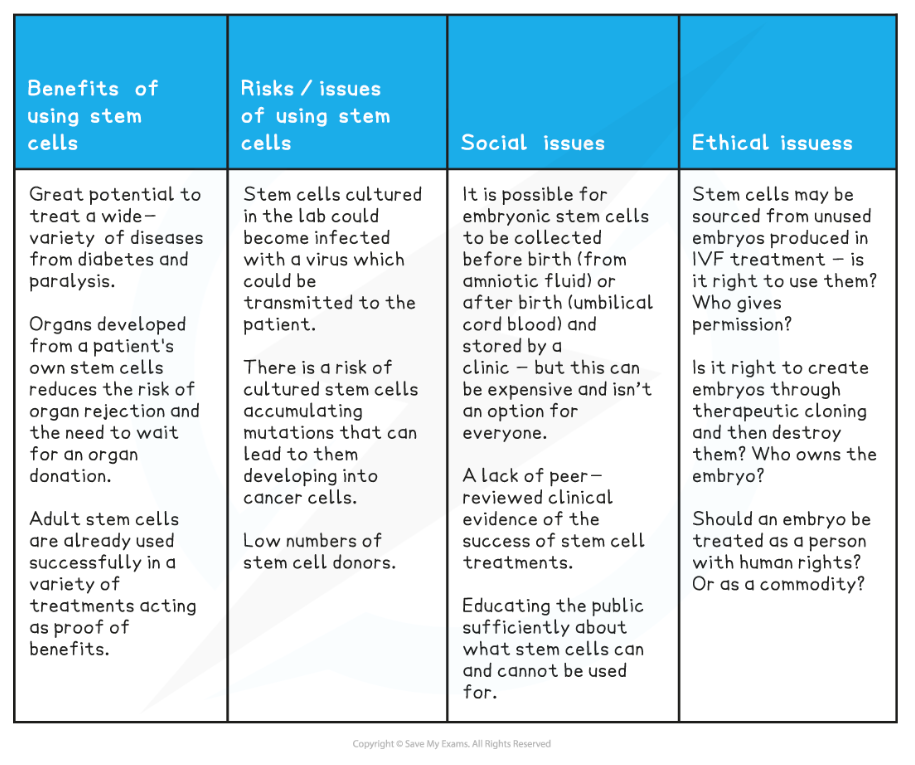
Evaluating the use of stem cells in medicine
- The use of stem cells that are collected from embryos created via IVF is ethically questionable as this results in a viable embryo (an embryo that could become a foetus if implanted in a uterus) being destroyed
- Although their use raises fewer ethical questions, the disadvantage of only using adult stem cells is that, unlike embryonic stem cells, they are unable to differentiate into all of the specialised cell types (some of which may be required to treat certain diseases)
- This means that society has to use all the available scientific knowledge to make decisions about the use of stem cells (especially embryonic stem cells) in medical therapies, considering all the arguments for and against their use
- Official regulatory authorities are required to help society make these decisions. They do this by comparing the benefits and ethical issues of stem cell research and making decisions on the extent to which stem cells can be used. These regulatory authorities carry out the following tasks:
- Reviewing proposals for scientific research that uses stem cells and deciding if this research should be allowed to go ahead
- Licensing and monitoring of research centres that are involved in stem cell research
- Providing guidelines and codes of practice for stem cell researchers to ensure they are working to the same high standards
- Monitoring developments in scientific research into stem cell therapies
- Providing governments and other professional bodies with correct, up-to-date advice and information on stem cell research, which in turn helps society to understand how stem cells are being used and why this work is important
Evaluating the Use of Stem Cells Table
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1