- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A (SNAB) A Level Biology:复习笔记1.2.7 Disaccharides
Disaccharides: Structure
- Two monosaccharides can join together via condensation reactions to form disaccharides
- A condensation reaction is one in which two molecules join together via the formation of a new chemical bond, with a molecule of water being released in the process
- The new chemical bond that forms between two monosaccharides is known as a glycosidic bond
- Common examples of disaccharides include
- Maltose
- Contains two molecules of glucose linked by a 1,4 glycosidic bond
- This means that the glycosidic bond is located between carbon 1 of one monosaccharide and carbon 4 of the other
- Contains two molecules of glucose linked by a 1,4 glycosidic bond
- Sucrose
- Contains a molecule of glucose and a molecule of fructose linked by a 1,2 glycosidic bond
- This means that the glycosidic bond is located between carbon 1 of one monosaccharide and carbon 2 of the other
- Contains a molecule of glucose and a molecule of fructose linked by a 1,2 glycosidic bond
- Lactose
- Contains a molecule of glucose and a molecule of galactose linked by a 1,4 glycosidic bond
- Maltose
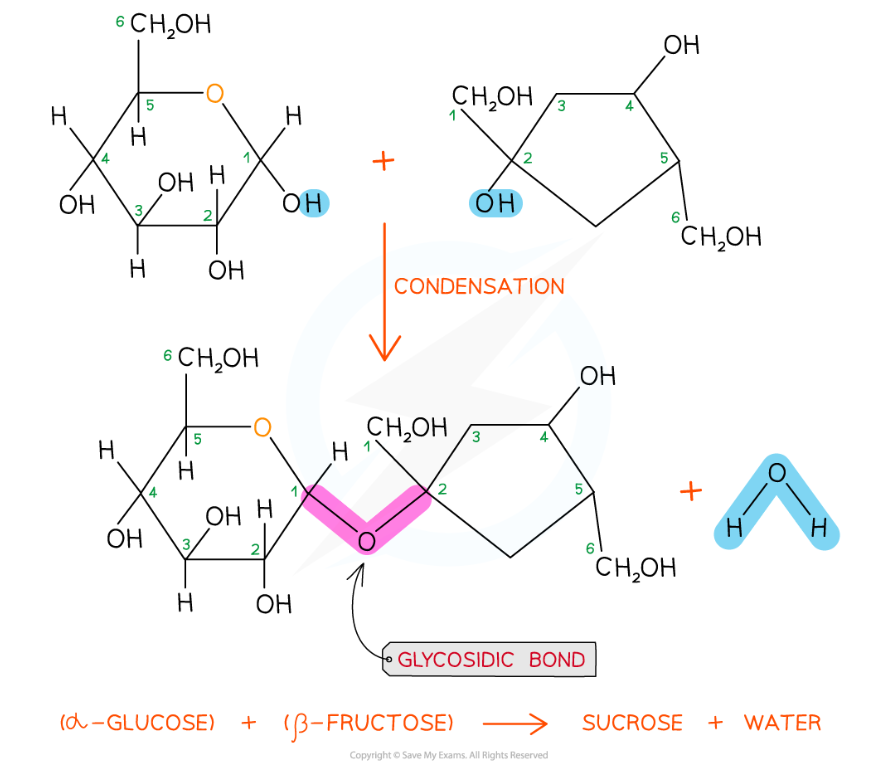
Sucrose is a disaccharide formed from a molecule of glucose (left) and a molecule of fructose (right) joined together by a 1,2 glycosidic bond
Disaccharides: Function
- The function of disaccharides is to provide the body with a quick-release source of energy
- Disaccharides are made up of two sugar molecules so they're easily broken down by enzymes in the digestive system into their respective monosaccharides and then absorbed into the bloodstream
- Due to the presence of a large number of hydroxyl groups, disaccharides are easily soluble in water
- These hydroxyl groups form hydrogen bonds with the water molecules when dissolved in aqueous solutions
- Just like monosaccharides they are sweet in taste
- Sucrose, also known as table sugar, is an example
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








