- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.5 Decay Equations
Edexcel IGCSE Physics: Double Science 复习笔记:7.1.5 Decay Equations
Decay Equations
- Radioactive decay events can be shown using a decay equation
- A decay equation is similar to a chemical reaction equation
- The particles present before the decay are shown before the arrow
- The particles produced in the decay are shown after the arrow
- During decay equations the sum of the mass and atomic numbers before the reaction must be the same as the sum of the mass and atomic numbers after the reaction
- The following decay equation shows Polonium-212 undergoing alpha decay
- It forms Lead-208 and an alpha particle
- An alpha particle can also be written as a helium nucleus (Symbol He)
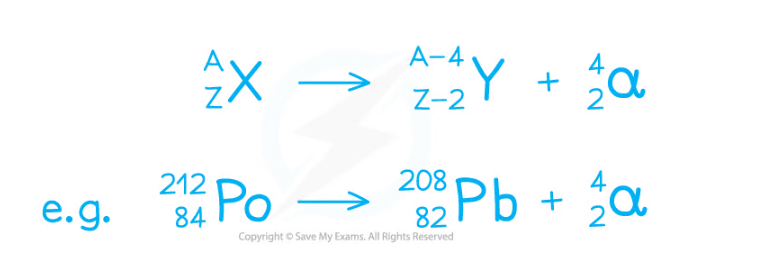
The polonium nucleus emits an alpha particle, causing its mass and charge to decrease. This means it changes into a new element
Alpha Decay
- During alpha decay an alpha particle is emitted from an unstable nucleus
- A completely new element is formed in the process
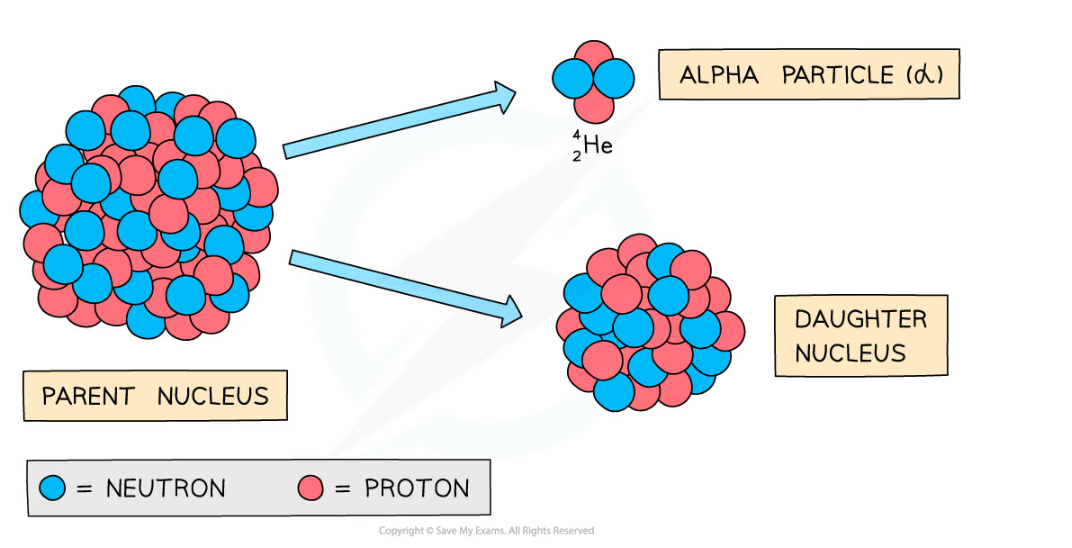
Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease
- An alpha particle is a helium nucleus
- It is made of 2 protons and 2 neutrons
- When the alpha particle is emitted from the unstable nucleus, the mass number and atomic number of the nucleus changes
- The mass number decreases by 4
- The atomic number decreases by 2
- The charge on the nucleus also decreases by 2
- This is because protons have a charge of +1 each
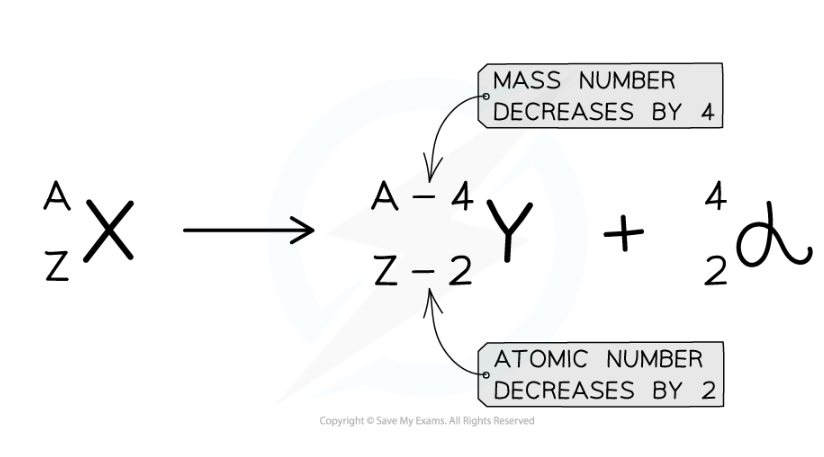
Alpha decay equation
Beta Decay
- During beta decay, a neutron changes into a proton and an electron
- The electron is emitted and the proton remains in the nuclei
- A completely new element is formed because the atomic number changes
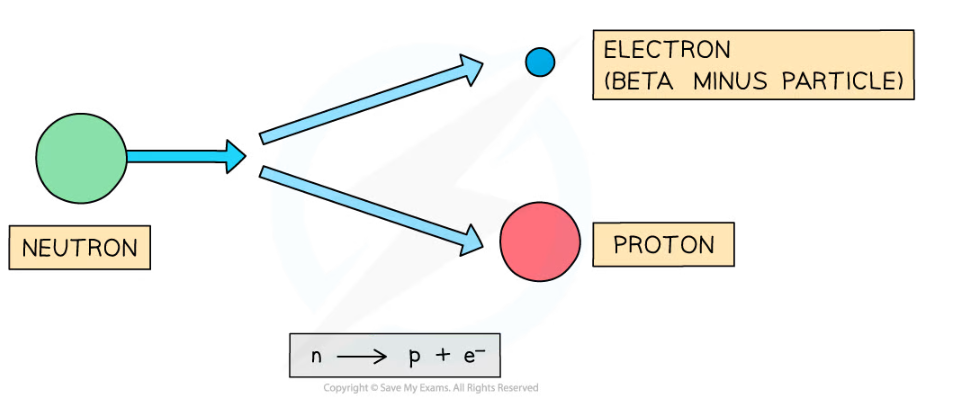
Beta decay often happens in unstable nuclei that have too many neutrons. The mass number stays the same, but the atomic number increases by one
- A beta particle is a high-speed electron
- It has a mass number of 0
- This is because the electron has a negligible mass, compared to neutrons and protons
- Therefore, the mass number of the decaying nuclei remains the same
- Electrons have an atomic number of -1
- This means that the new nuclei will increase its atomic number by 1 in order to maintain the overall atomic number before and after the decay
- The following equation shows carbon-14 undergoing beta decay
- It forms nitrogen-14 and a beta particle
- Beta particles are written as an electron in this equation
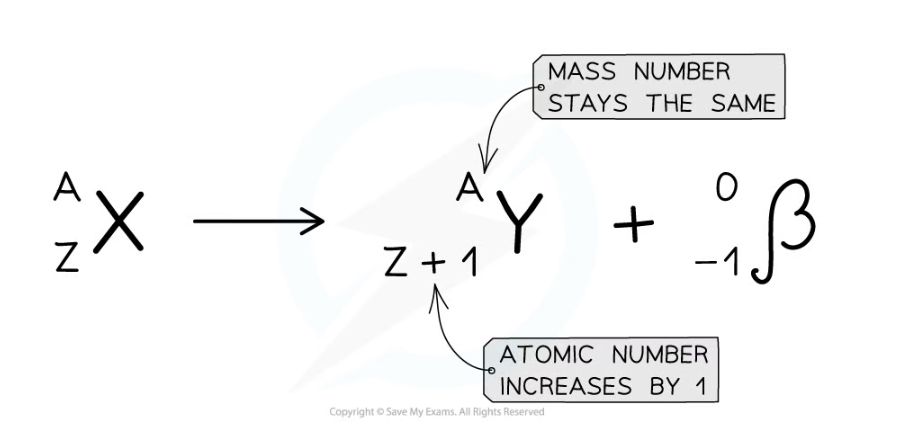
Beta decay equation
Gamma Decay
- During gamma decay, a gamma ray is emitted from an unstable nucleus
- The process that makes the nucleus less energetic but does not change its structure
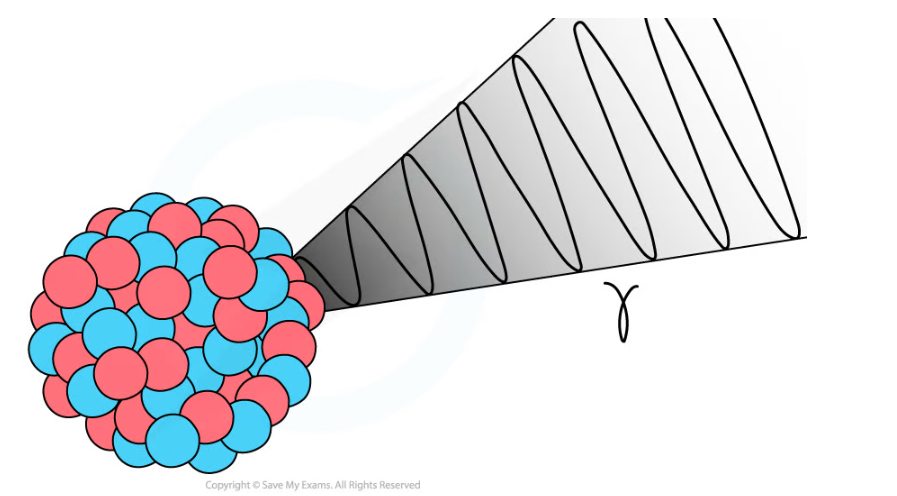
Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus
- The gamma ray that is emitted has a lot of energy, but no mass or charge
- Here is an example of Uranium-238 undergoing gamma decay
- Notice that the mass number and atomic number of the unstable nuclei remains the same during the decay
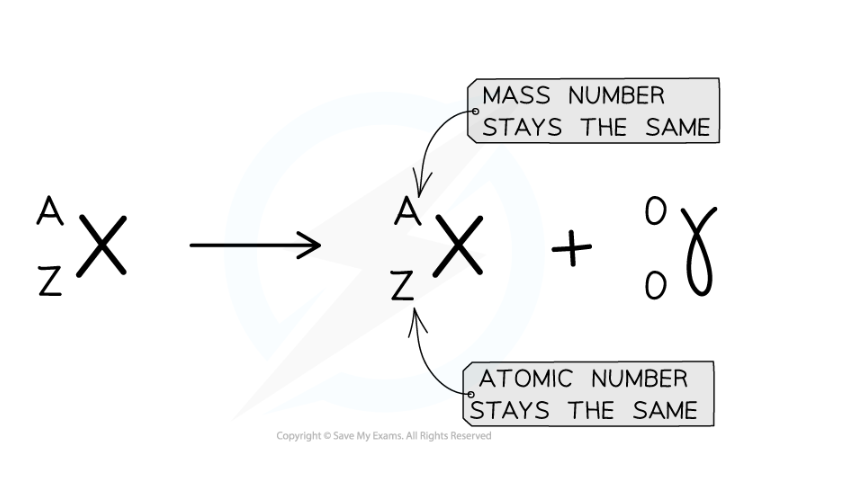
Gamma decay equation
Neutron Emission
- A small number of isotopes can decay by emitting neutrons.
- When a nucleus emits a neutron:
- The number of protons does not change:
The atomic (proton) number does not change
- The total number of particles (nucleons) in the nucleus decreases by 1:
- The mass (nucleon) number decreases by 1
- The number of protons does not change:
Worked Example
A nucleus with 84 protons and 126 neutrons undergoes alpha decay. It forms lead, which has the element symbol Pb. Which of the isotopes of lead pictured is the correct one formed during the decay?
Which of the isotopes of lead pictured is the correct one formed during the decay?
ANSWER: A
Step 1: Calculate the mass number of the original nucleus
-
- The mass number is equal to the number of protons plus the number of neutrons
- The original nucleus has 84 protons and 126 neutrons
84 + 126 = 210
-
- The mass number of the original nucleus is 210
Step 2: Calculate the new atomic number
-
- The alpha particle emitted is made of two protons and two neutrons
- Protons have an atomic number of 1, and neutrons have an atomic number of 0
- Removing two protons and two neutrons will reduce the atomic number by 2
84 – 2 = 82
-
- The new nucleus has an atomic number of 82
Step 3: Calculate the new mass number
-
- Protons and neutrons both have a mass number of 1
- Removing two protons and two neutrons will reduce the mass number by 4
210 – 4 = 206
-
- The new nucleus has a mass number of 206
Worked Example
A nucleus with 11 protons and 13 neutrons undergoes beta decay. It forms magnesium, which has the element symbol Mg. Which is the correct isotope of magnesium formed during the decay?
Which is the correct isotope of magnesium formed during the decay?
ANSWER: D
Step 1: Calculate the mass number of the original nucleus
-
- The mass number is equal to the number of protons plus the number of neutrons
- The original nucleus has 11 protons and 13 neutrons
11 + 13 = 24
-
- The mass number of the original nucleus is 24
Step 2: Calculate the new atomic number
-
- During beta decay a neutron changes into a proton and an electron
- The electron is emitted as a beta particle
- The neutron has an atomic number of 0 and the proton has an atomic number of 1
- So the atomic number increases by 1
11 + 1 = 12
-
- The new nucleus has an atomic number of 12
Step 3: Calculate the new mass number
-
- Protons and neutrons both have a mass number of 1
- Changing a neutron to a proton will not affect the mass number
- The new nucleus has a mass number of 24 (the same as before)
Exam Tip
It is easy to forget that an alpha particle is a helium nucleus. The two are interchangeable, so don’t be surprised to see either used in the exam. You are not expected to know the names of the elements produced during radioactive decays, but you do need to be able to calculate the mass and atomic numbers by making sure they are balanced on either side of the reaction.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








