Edexcel IGCSE Physics: Double Science 复习笔记:4.1.5 Core Practical: Investigating Thermal Energy
Core Practical 8: Investigating Thermal Energy
Equipment List
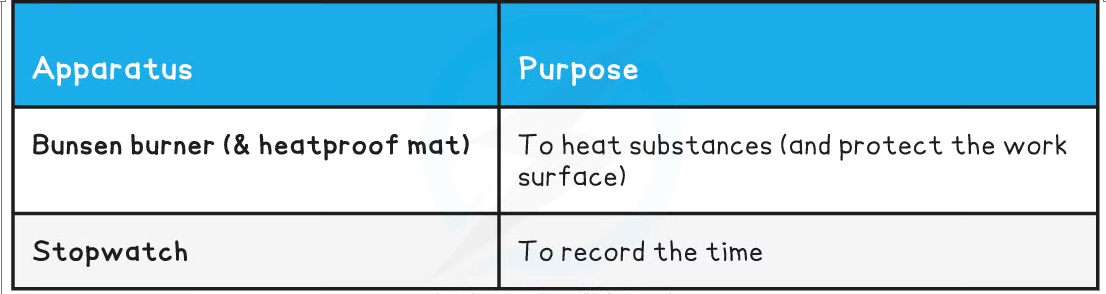
Resolution of measuring equipment:
- Thermometer = 1°C
- Stopwatch = 0.01 s
Experiment 1: Investigating Conduction
Aim of the Experiment
- The aim of the experiment is to investigate the rate of conduction in four different metals
Variables:
- Independent variable = Type of metal
- Dependent variable = Rate of conduction
- Control variables:
- Size and thickness of metal strips
- Amount of wax used
- Identical ball bearings
Method
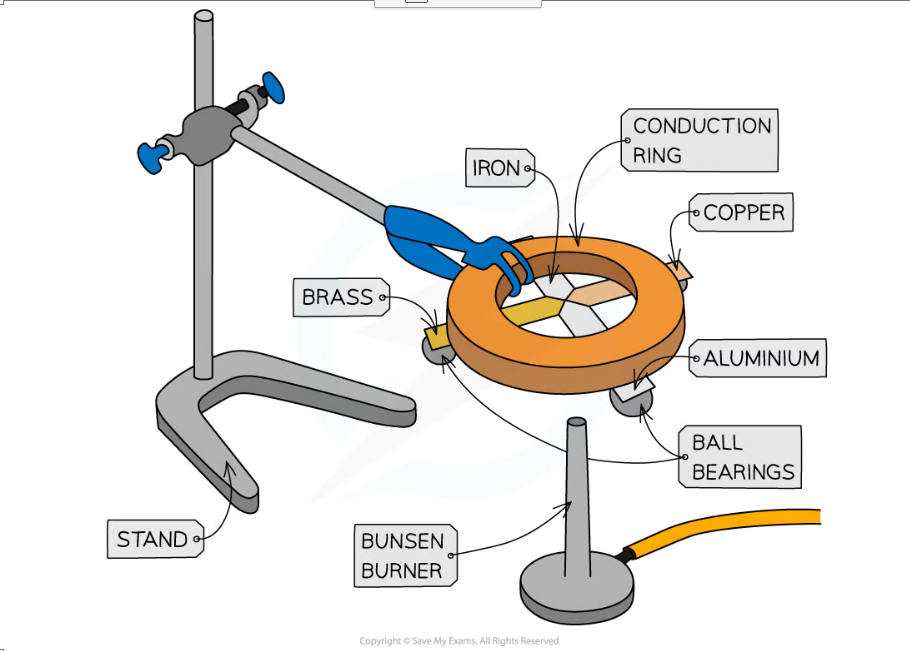
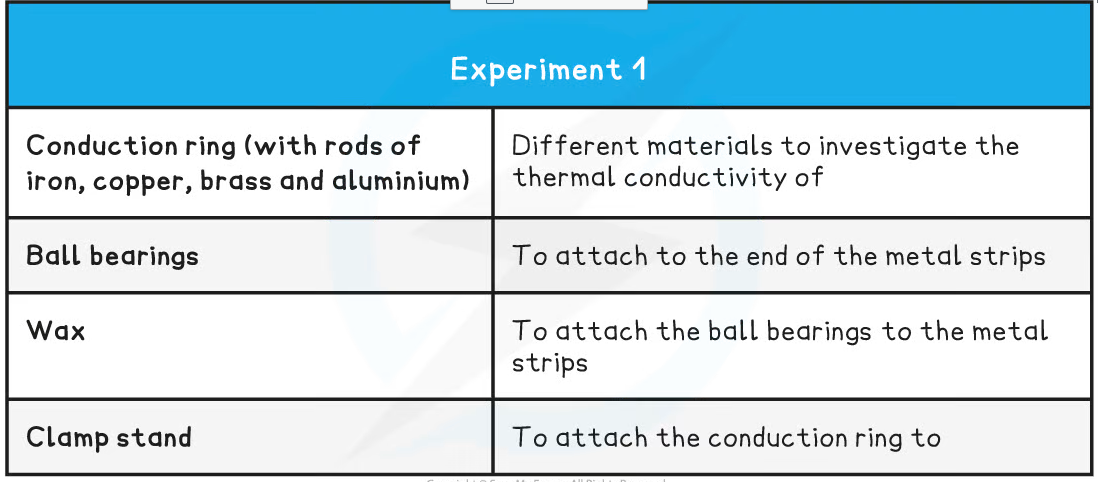
The above apparatus consists of 4 different metal strips of equal width and length arrange around an insulated circle
- Attach ball bearings to the ends of each metal strip at an equal distance from the centre, using a small amount of wax
- The strips should then be turned upside down and the centre heated gently using a bunsen burner so that each of the strips is heated at the central point where they meet
- When the heat is conducted along to the ball bearing, the wax will melt and the ball bearing will drop
- Time how long this takes for each of the strips and record in a table
- Repeat the experiment and calculate an average of each time
Analysis of Results
- Order the metals according to their conductivity
- The first ball bearing to fall will be from the rod that is the best conductor
- This is because materials with high thermal conductivity heat up faster than materials with low thermal conductivity
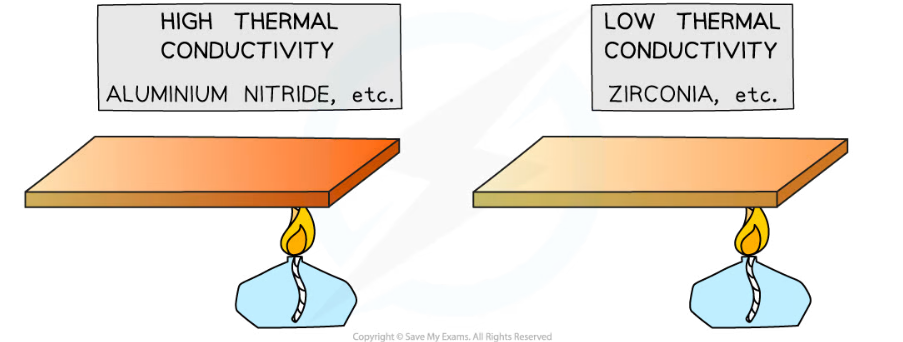
Examples of materials with high and low thermal conductivity
- The results should show the conductivity ranked from highest to lowest is:
- Copper (fastest time for ball bearing to fall)
- Aluminium
- Brass
- Iron (slowest time for ball bearing to fall)
Experiment 2: Investigating Convection
Aims of the Experiment
- The aim of the experiment is to investigate the rate of convection of potassium permanganate crystals in two different temperatures of water
Variables:
- Independent variable = Temperature of water
- Dependent variable = Rate of convection
- Control variables:
- Amount of water in beaker
- Size of bunsen burner flame
- Size of potassium permanganate crystal
Method
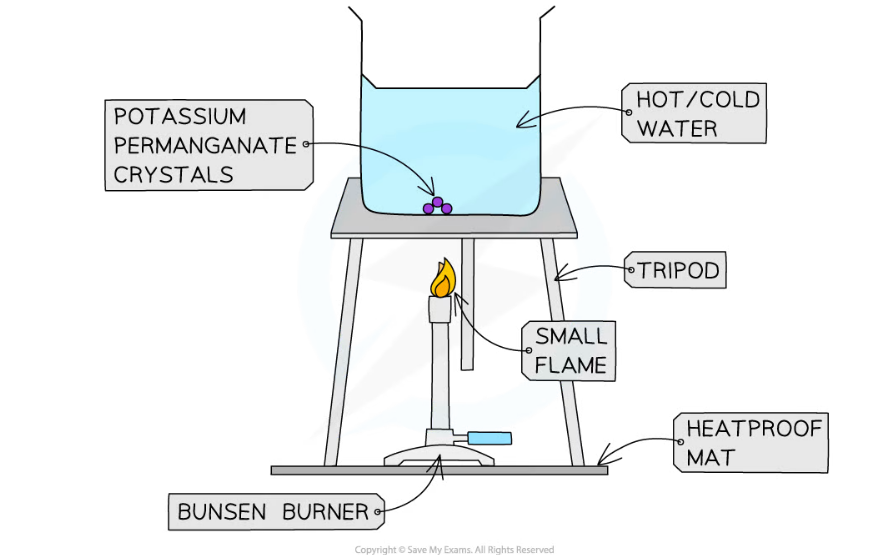
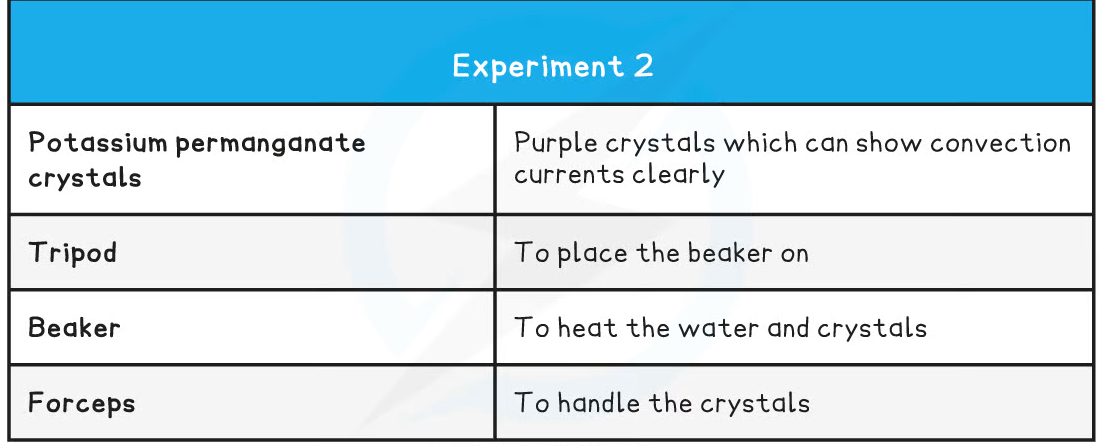
Apparatus used to investigate potassium permanganate crystals undergoing convection in water
- Fill the beaker with cold water (not too full) and place it on top of a tripod and heatproof mat
- Pick up the crystal using forceps and drop it into the centre of the beaker – do this carefully to ensure the crystal does not dissolve prematurely
- Heat the beaker using the Bunsen burner and record observations
- Repeat experiment with hot water and record observations
Analysis of Results
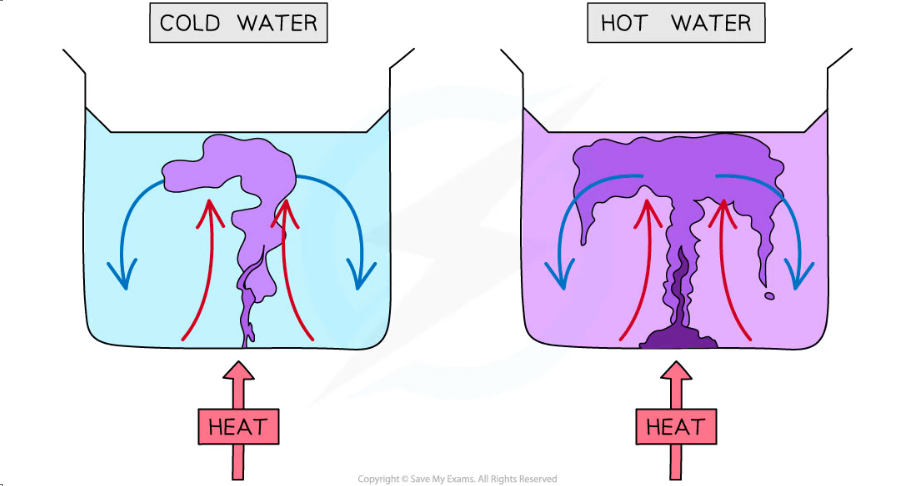
- Heat is initially transferred through the glass wall of the beaker by conduction
- The water in the region of the Bunsen flame is heated and expands, becomes less dense and rises
- This causes the dissolved purple crystal to flow up with the water
- Meanwhile, when the water at the top of the beaker cools, it becomes denser again and falls
- The process continues which leads to a convection current where heat is transferred through the liquid
- The dissolved purple crystal follows this current which is what is observed during this experiment
- It should be observed that the convection current is faster in hot water
- This is because the higher the temperature, the higher the kinetic energy of the water molecules
- Therefore, in hot water, the molecules of potassium permanganate move around the beaker faster
Experiment 3: Investigating Radiation
Aims of the Experiment
The aim of the experiment is to investigate how the amount of infrared radiation absorbed or radiated by a surface depends on the nature of that surfaceVariables:
- Independent variable = Colour
- Dependent variable = Temperature
- Control variables:
- Identical flasks (except for their colour)
- Same amounts of hot water
- Same starting temperature of the water
- Same time interval
Method
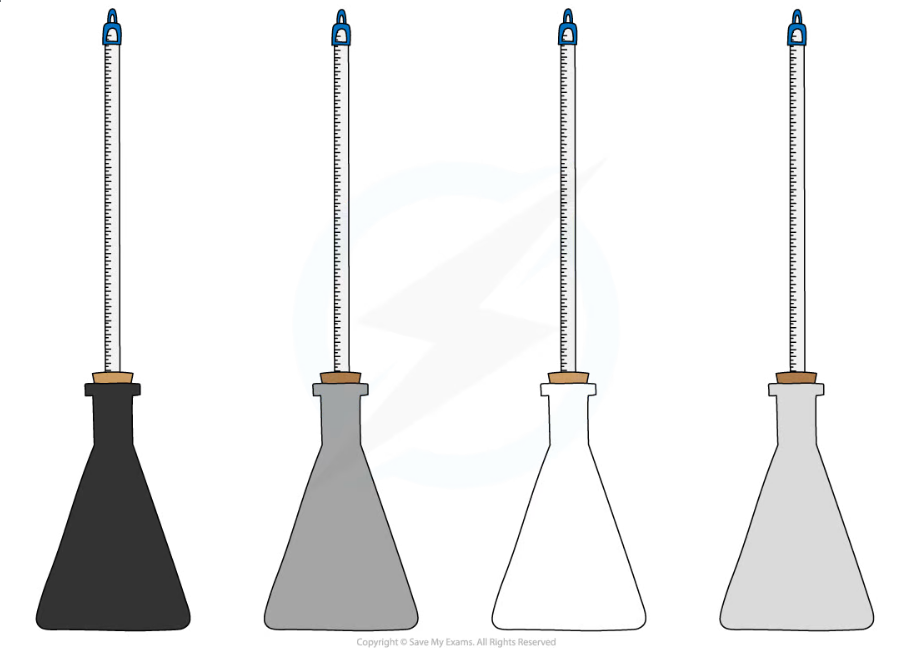
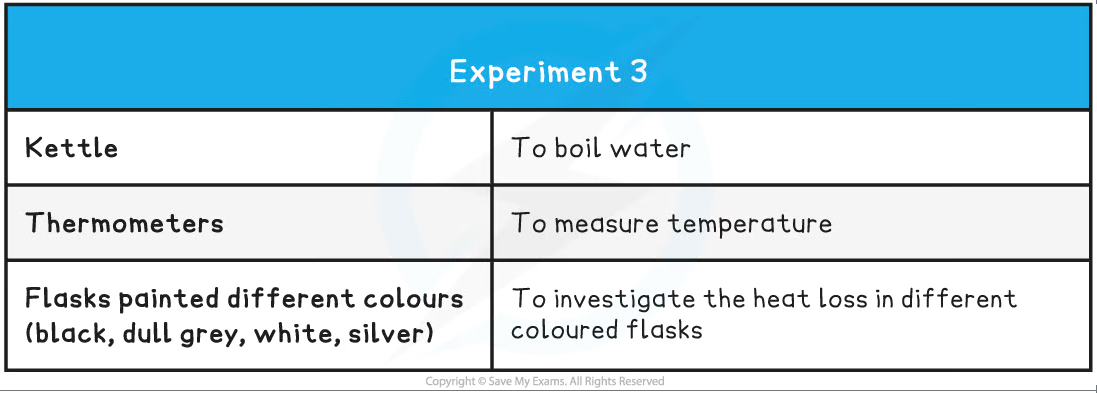
Different coloured beakers for investigating infrared radiation apparatus
- Set up the four identical flasks painted black, grey, white and silver
- Fill the flasks with hot water, ensuring the measurements start from the same initial temperature
- Note the starting temperature, then measure the temperatures at regular intervals e.g. every 30 seconds for 10 minutes
- An example table of results might look like this:
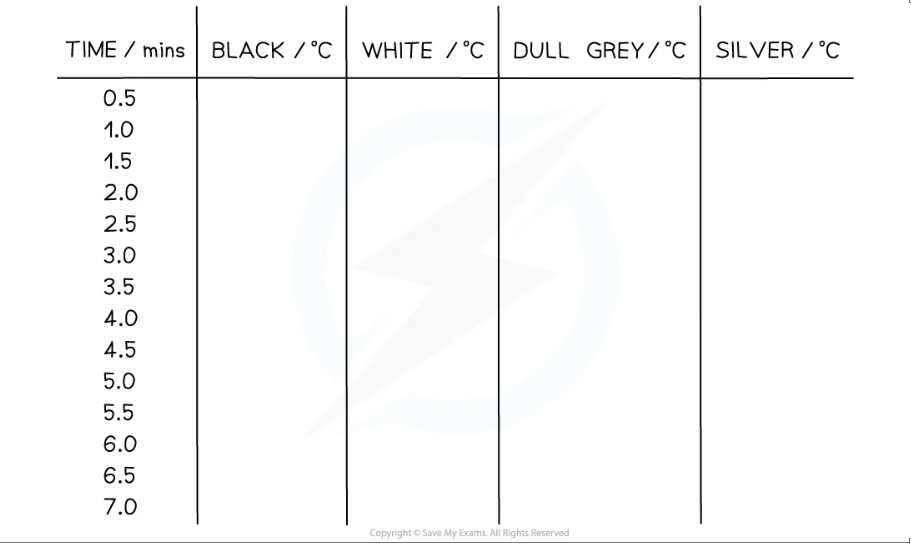
Analysis of Results
- All warm objects emit thermal radiation in the form of infrared waves
- The intensity (and wavelength) of the emitted radiation depends on:
- The temperature of the body (hotter objects emit more thermal radiation)
- The surface area of the body (a larger surface area allows more radiation to be emitted)
- The colour of the surface
- Most of the heat lost from the beakers will be due to conduction and convection
- This will be the same for each beaker, as colour does not affect heat loss in this way
- Any difference in heat loss between the beakers must, therefore, be due to infrared (thermal) radiation
- To compare the rate of heat loss of each flask, plot a graph of temperature on the y-axis against time on the x-axis and draw curves of best fit
- The expected results are shown on the graph below:
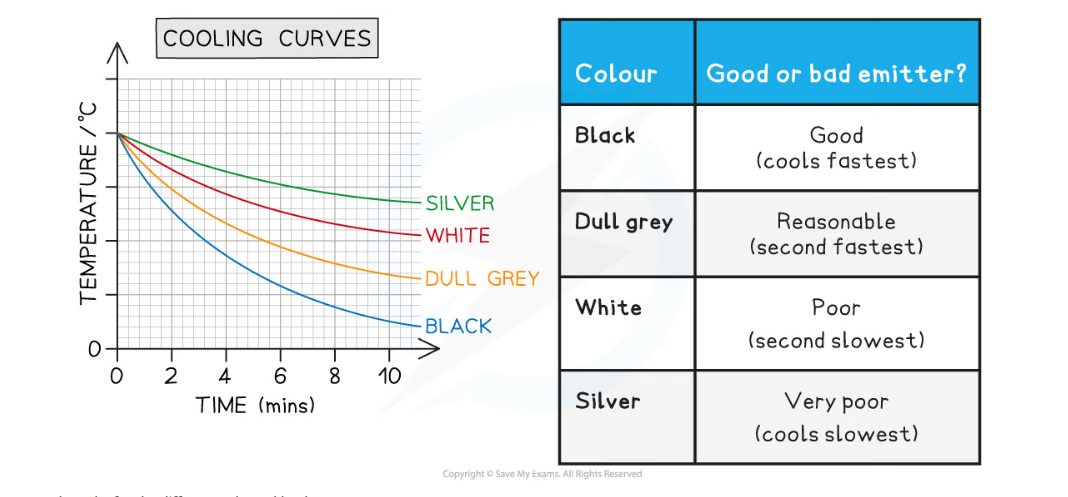
Example graph and table of the expected results for the different coloured beakers
Evaluating the Experiments
Systematic Errors:
- For experiment 1:
- Allow the rods to cool to room temperature before heating so that they all begin at the same temperature
- For experiment 3:
- Make sure the starting temperature of the water is the same for each material since this will cool very quickly
- It is best to do this experiment in pairs to coordinate starting the stopwatch and immersing the thermometer
- Use a data logger connected to a digital thermometer to get more accurate readings
Random Errors:
- For experiment 1:
- Avoid handling the rods and the wax too much before heating
- For experiment 3:
- Make sure the hole for the thermometer isn’t too big, otherwise the heat will escape through the hole
- Take repeated readings for each coloured flask
- Read the values on the thermometer at eye level, to avoid parallax error
Safety Considerations
- Safety goggles should be worn when using a Bunsen burner
- Ensure the safety (orange) flame is on when the Bunsen burner is not heating anything
- Potassium permanganate in its solid form is an oxidiser, harmful if swallowed and harmful to aquatic life
- Keep water away from all electrical equipment
- Make sure not to touch the hot water directly
- Run any burns immediately under cold running water for at least 5 minutes
- Do not overfill the kettle
- Make sure all the equipment is in the middle of the desk, and not at the end to avoid knocking over the beakers
- Carry out the experiments only whilst standing, in order to react quickly to any spills or burns










