- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Physics:复习笔记11.7 Nuclear Decay Equations
Nuclear Decay Equations
Alpha Decay
- An alpha particle consists of 2 protons and 2 neutrons
(It is emitted from large unstable nuclei)
- When an alpha particle is emitted from a nucleus:
- The nucleus loses 2 protons:
The proton (atomic) number decreases by 2
- The nucleus loses 4 particles (nucleons) in total:
The nucleon (mass) number decreases by 4
- The nucleus loses 2 protons:
- Equation for alpha emission:
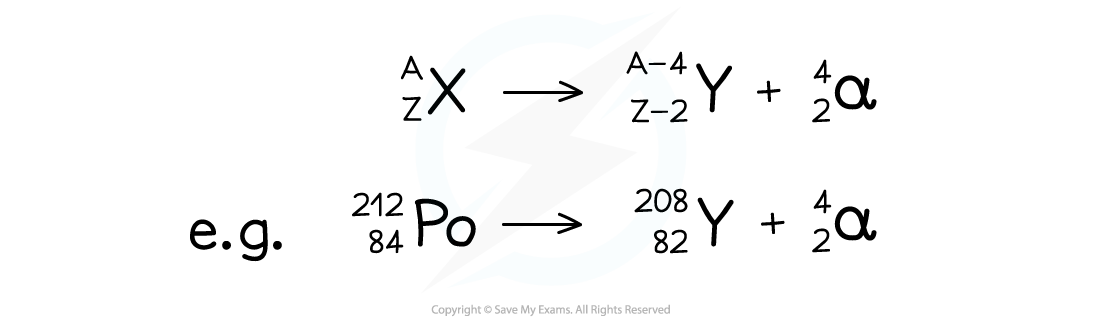
- Nuclear equations, just like chemical equations, balance:
- The sum of the upper (mass) numbers on the left of each equation should equal the sum on the right
- The sum of the lower (atomic) numbers should also balance
Beta Emission
- Equation for beta emission:
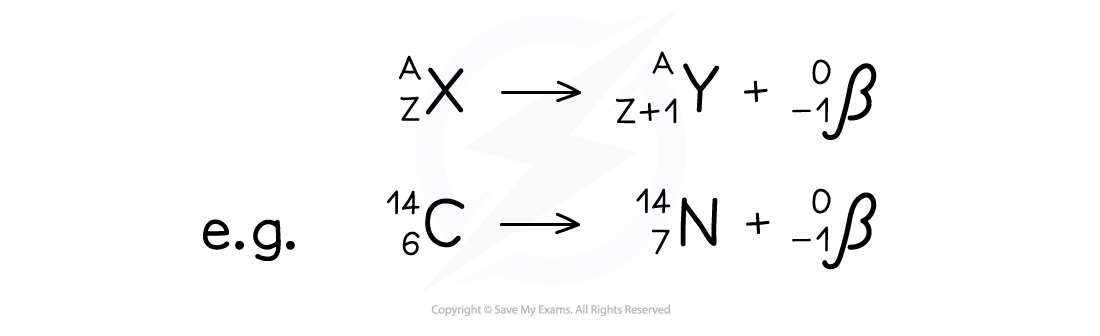
- Note that the beta particle is given an atomic number of -1 in the above examples
This is because the atomic number is being used to measure charge in this case:Protons, being positive particles, have positive atomic numbers
Electrons, being negative, have a negative number
Gamma Decay
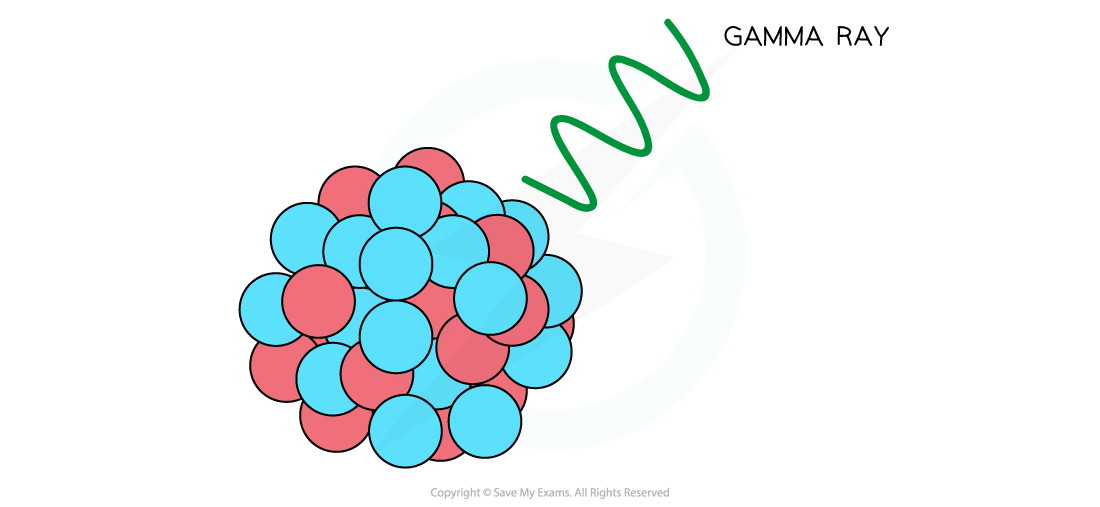
- During gamma decay, a gamma ray is emitted from an unstable nucleus
- The process that makes the nucleus less energetic but does not change its structure
Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus
- The gamma ray that is emitted has a lot of energy, but no mass or charge
- Here is an example of Uranium-238 undergoing gamma decay
- Notice that the mass number and atomic number of the unstable nuclei remains the same during the decay
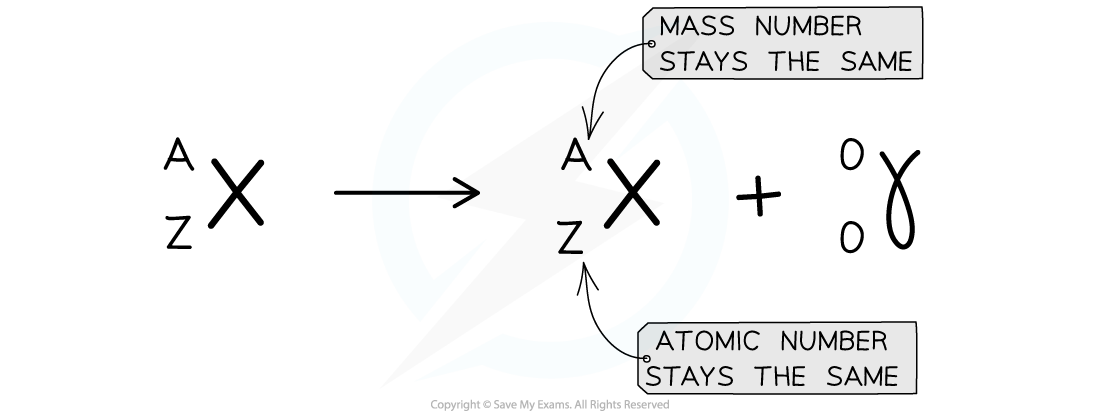
Gamma decay equation

An example of Barium decay through the release of a gamma ray
Worked Example
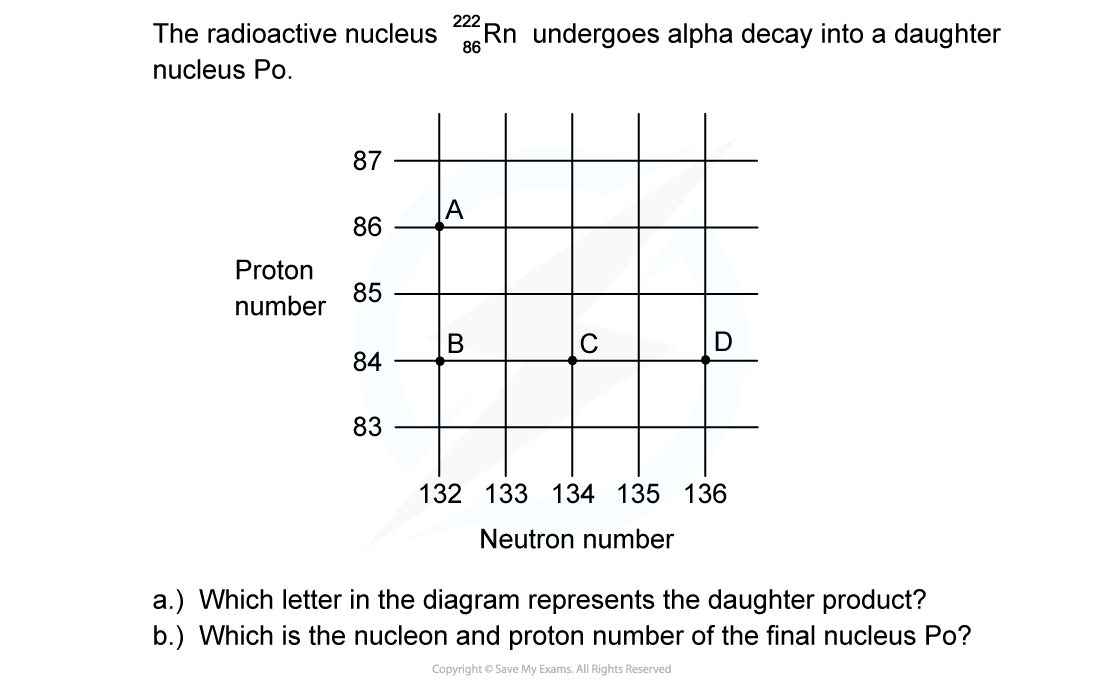
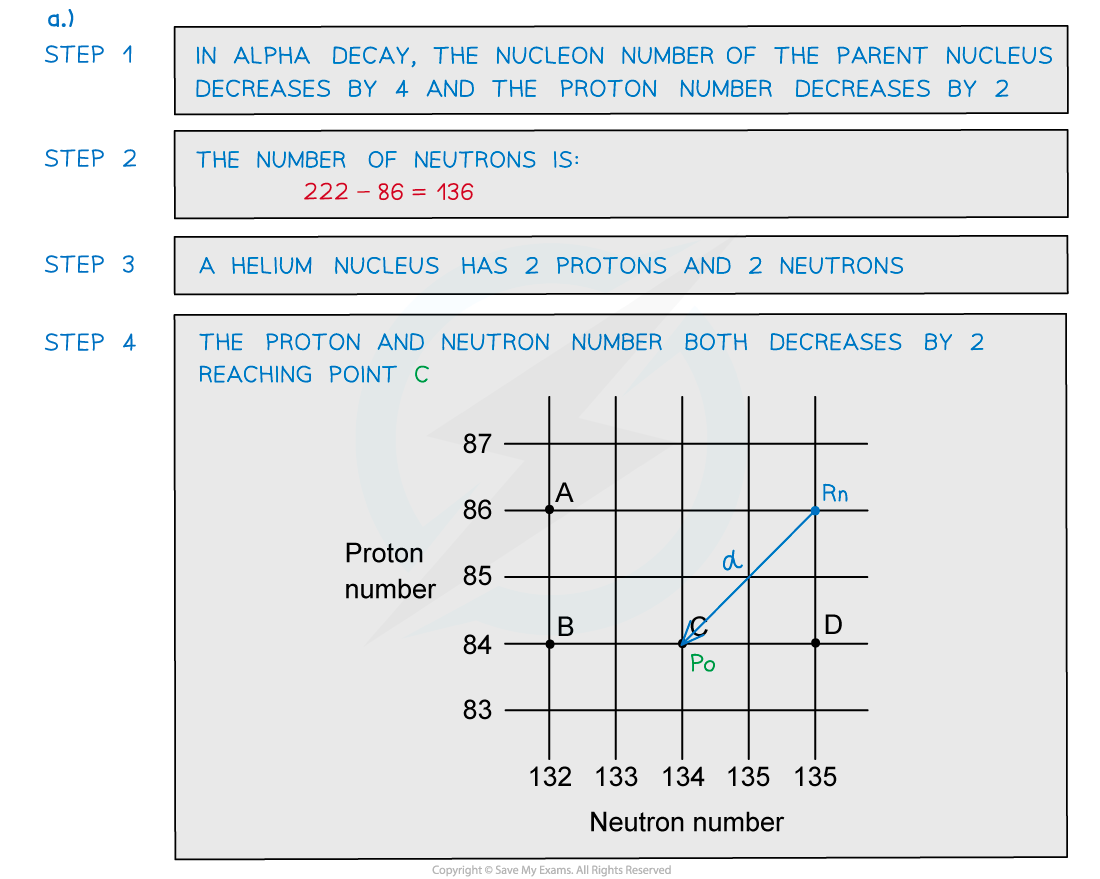
ANSWER: C
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









