- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Physics:复习笔记9.8 Core Practical 14: Investigating Gas Pressure & Volume
Investigating Gas Pressure and Volume
- Boyle's Law relates pressure and volume at constant temperature
- This investigation is one valid example of how this required practical might be tackled, others do exist
Variables
- Independent variable = Mass, m (kg)
- Dependent variable = Volume, V (m3)
- Control variables:
- Temperature
- Cross-sectional area of the syringe
Equipment List
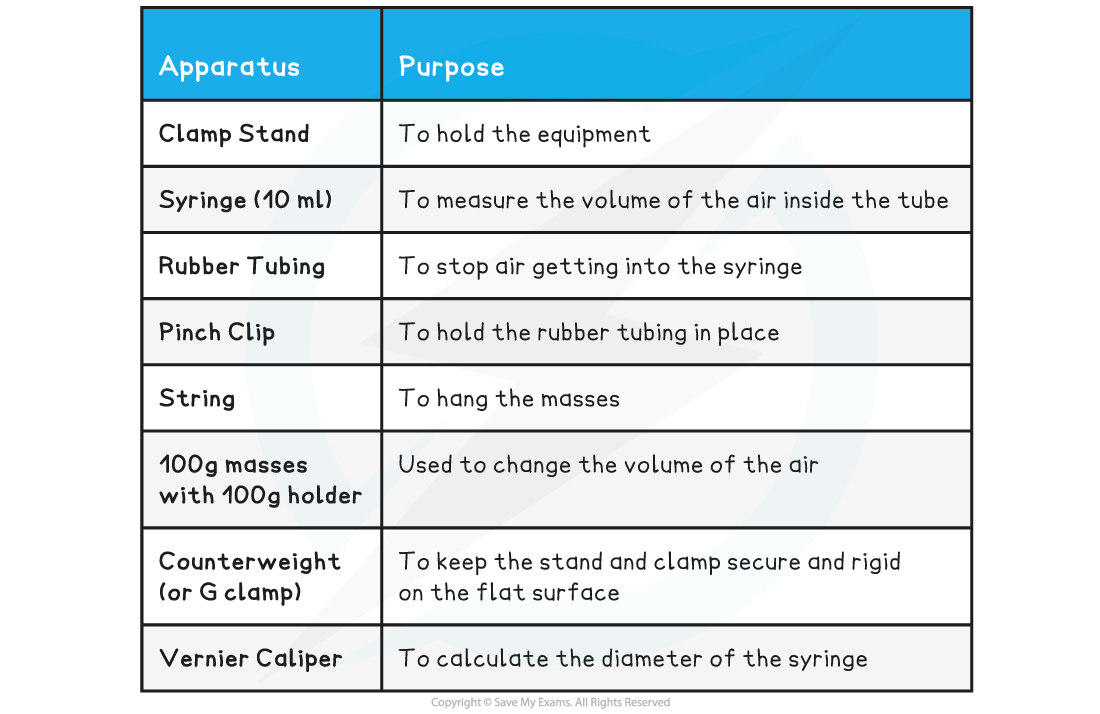
- Resolution of measuring equipment:
- Pressure gauge = 0.02 × 105 Pa
- Volume = 0.2 cm3
- Vernier Caliper = 0.02 mm
Method
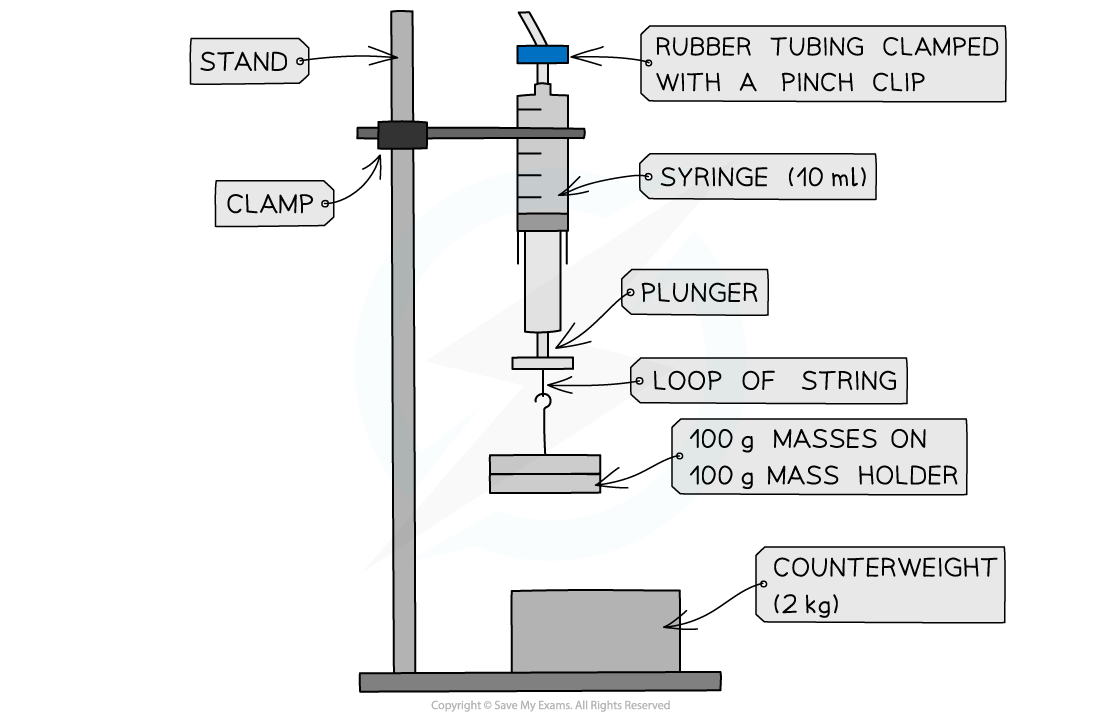
Apparatus setup for Boyle’s Law
- With the plunger removed from the syringe, measure the inside diameter, d of the syringe using a Vernier caliper.
- Take 3 readings and find an average
- The plunger should be replaced and the rubber tubing should be fit over the nozzle and clamped with a pinch clip as close to the nozzle as possible
- Set up the apparatus as shown in the diagram
- Push the syringe upwards until it reads the lowest volume of air visible. Record this volume
- Add the 100 g mass holder to the loop of string at the bottom of the plunger. Wait a few seconds before taking the reading (this allows temperature to equilibrate after work is done against the plunger when the volume increases)
- Record the value of the new volume from the syringe scale
- Repeat the experiment by adding 100 g masses and recording the readings up to 10 readings.
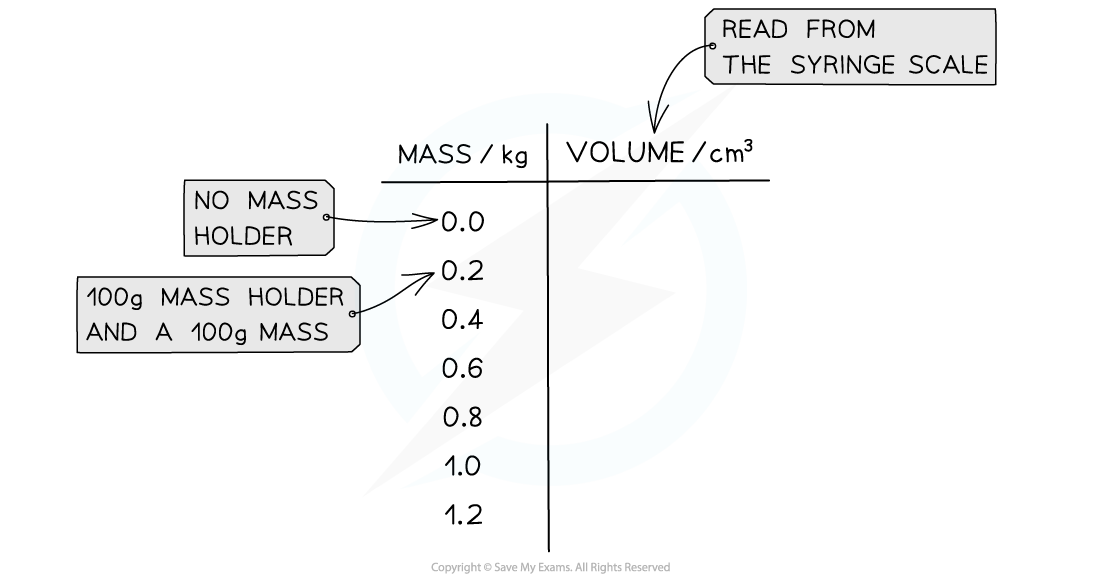
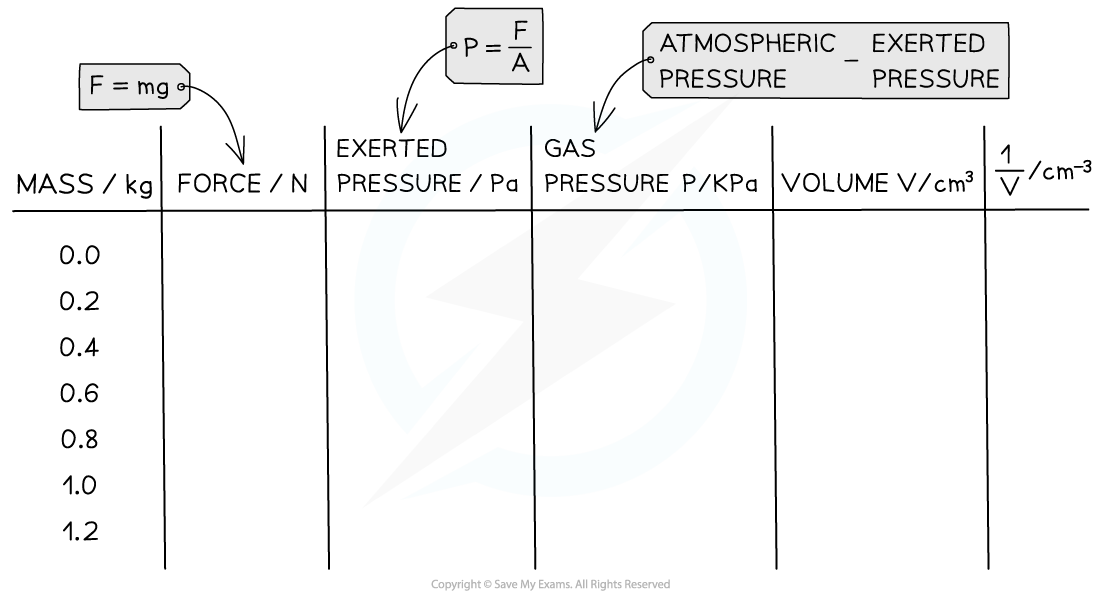
- An example table of results might look like this:

Analysing the Results
- Boyle’s Law can be represented by the equation:
pV = constant
- This means the pressure must be calculated from the experiment
- The exerted pressure of the masses is calculated by:
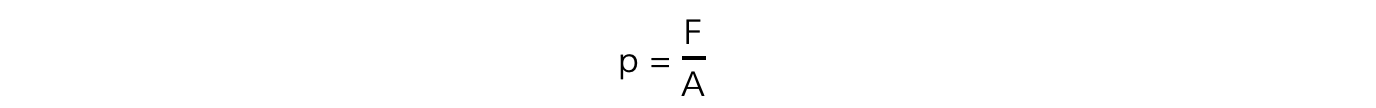
- Where:
- F = weight of the masses, mg (N)
- A = cross-sectional area of the syringe (m2)
- The cross-sectional area is found from the equation for the area of a circle:
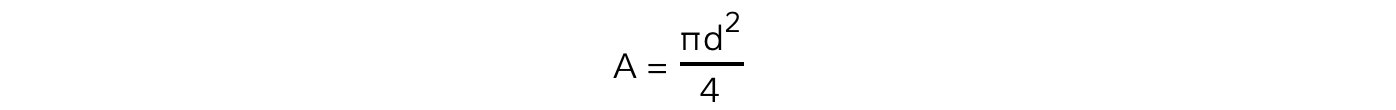
- To calculate the pressure of the gas:
Pressure of the gas = Atmospheric pressure – Exerted pressure from the masses
- Where:
- Atmospheric pressure = 101 kPa
- The table of results may need to be modified to fit these extra calculations. Here is an example of how this might look:
- Once these values are calculated:
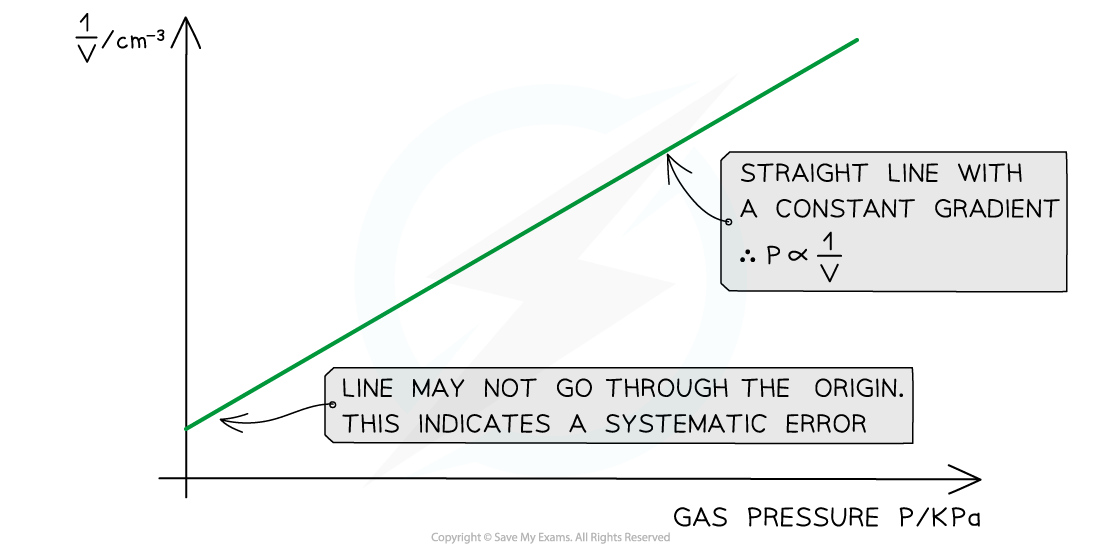
- Plot a graph of p against 1 / V and draw a line of best fit
- If this plot is a straight line graph, this means that the pressure is proportional to the inverse of the volume, hence confirming Boyle's Law (pV = constant)
Evaluating the Experiment
Systematic Errors:
- There may be friction in the syringe which causes a systematic error
- Use a syringe that has very little friction or lubricated it, so the only force is from the weights pulling the syringe downwards
Random Errors:
- The reading of the volume should be taken a few seconds after the mass has been added to the holder to allow temperature changes to equilibrate
- Room temperature must be kept constant
Safety Considerations
- A counterweight or G-clamp must be used to avoid the stand toppling over and causing injury, especially if the surface is not completely flat
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








