- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Physics:复习笔记9.7 Ideal Gas Equation
Ideal Gas Equation
- When calculating for gases, assume that the gas is an ideal gas
- The three gas laws (explained below) can be combined to create one equation in terms of pressure, volume, temperature and amount of gas.
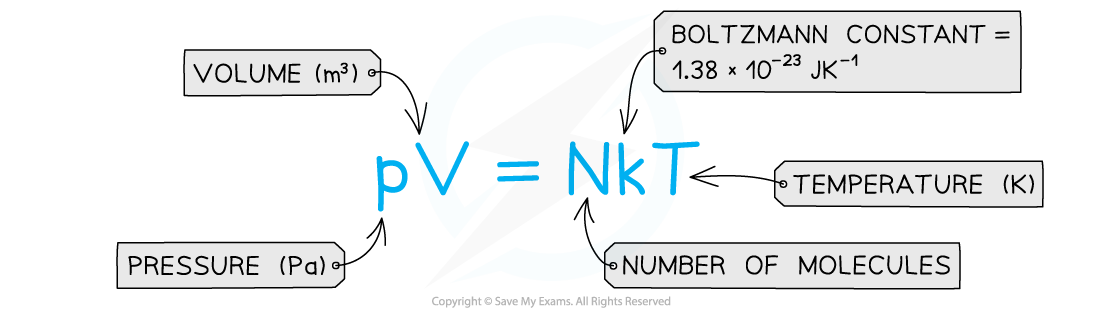
The Boltzmann Constant, k
- The Boltzmann constant k is used in the ideal gas equation and is defined as:
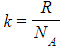
- Where:
- R = molar gas constant
- NA = Avogadro’s constant
- Boltzmann’s constant therefore has a value of

- The Boltzmann constant relates the properties of microscopic particles (e.g. kinetic energy of gas molecules) to their macroscopic properties (e.g. temperature)
- This is why the units are J K-1
- Its value is very small because the increase in kinetic energy of a molecule is very small for every incremental increase in temperature
The Gas Laws
- The ideal gas laws are the experimental relationships between pressure (P), volume (V) and temperature (T) of an ideal gas
- The mass and the number of molecules of the gas is assumed to be constant for each of these quantities
Boyle’s Law
- If the temperature T of an ideal gas is constant, then Boyle’s Law is given by:
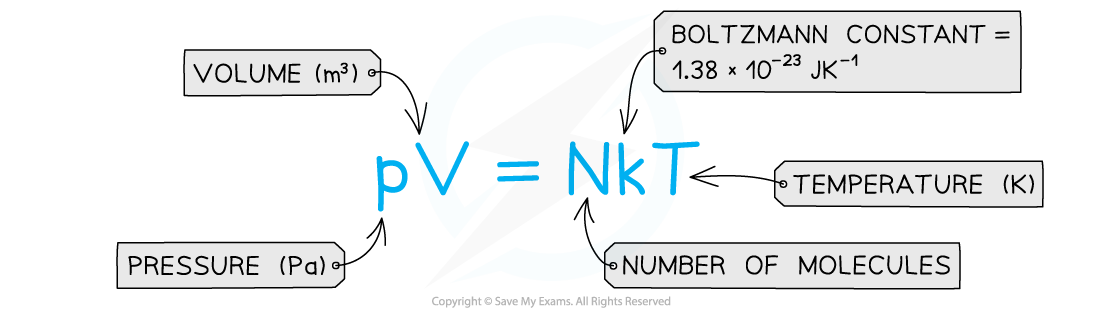
- This means the pressure is inversely proportional to the volume of a gas
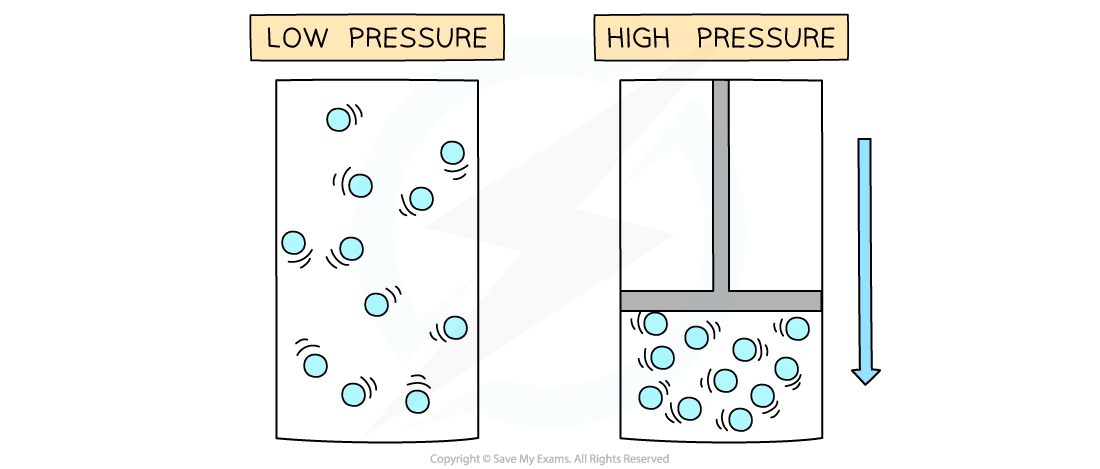
Pressure increases when a gas is compressed
- The relationship between the pressure and volume for a fixed mass of gas at constant temperature can also be written as:
P1V1 = P2V2
- Where:
- P1 = initial pressure (Pa)
- P2 = final pressure (Pa)
- V1 = initial volume (m3)
- V2 = final volume (m3)
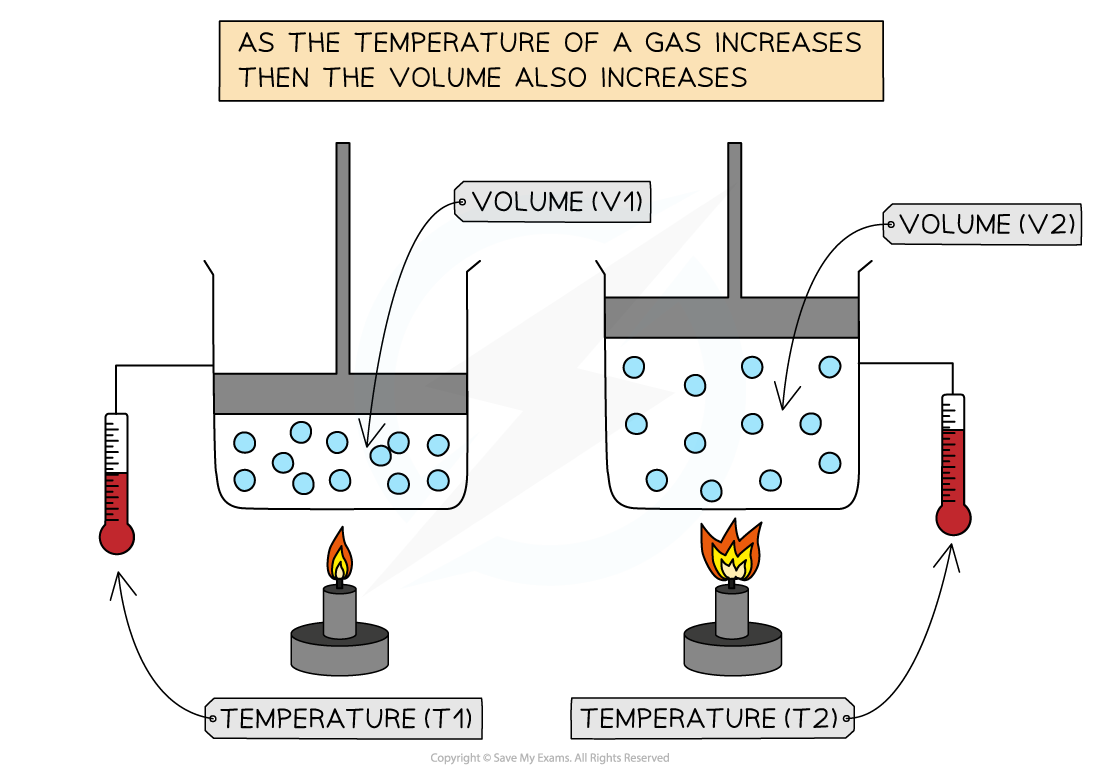
Charles's Law
- If the pressure P of an ideal gas is constant, then Charles’s law is given by:
V ∝ T
- This means the volume is proportional to the temperature of a gas
- The relationship between the volume and thermodynamic temperature for a fixed mass of gas at constant pressure can also be written as:
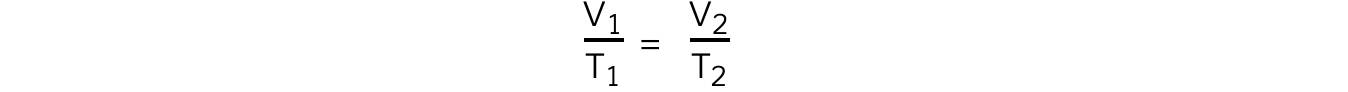
-
- Where:
- V1 = initial volume (m3)
- V2 = final volume (m3)
- T1 = initial temperature (K)
- T2 = final temperature (K)
- Where:
Pressure Law
- If the volume V of an ideal gas is constant, the Pressure law is given by:
P ∝ T
- This means the pressure is proportional to the temperature
- The relationship between the pressure and thermodynamic temperature for a fixed mass of gas at constant volume can also be written as:
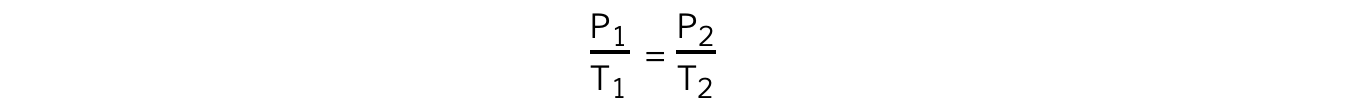
- Where:
- P1 = initial pressure (Pa)
- P2 = final pressure (Pa)
- T1 = initial temperature (K)
- T2 = final temperature (K)

Worked Example
A storage cylinder of an ideal gas has a volume of 8.3 × 103 cm3. The gas is at a temperature of 15oC and a pressure of 4.5 × 107 Pa. Calculate the amount of gas in the cylinder, in moles.
Step 1: Write down the ideal gas equation
Since the number of moles (n) is required, use the equation:
![]()
Step 2: Rearrange the equation for the number of moles n
![]()
Step 3: Substitute in values
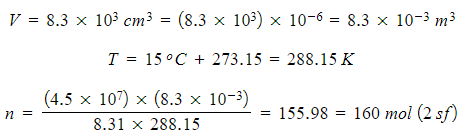
Exam Tip
After you solve a problem using any of the gas laws (or all of them combined), always check whether your final result makes physical sense - e.g. if you are asked to calculate the final pressure of a fixed mass of gas being heated at constant volume, your result must be greater than the initial pressure given in the problem (since Gay- Lussac's law states that pressure and absolute temperature are directly proportional at constant volume).
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









