- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Physics:复习笔记9.6 Kinetic Theory of Gases Equation
Kinetic Theory of Gases Equation
- Gases consist of atoms or molecules randomly moving around at high speeds
- The kinetic theory of gases models the thermodynamic behaviour of gases by linking the microscopic properties of particles (mass and speed) to macroscopic properties of particles (pressure and volume)
- The theory is based on the following assumptions:
- Molecules of gas behave as identical, hard, perfectly elastic spheres
- The volume of the molecules is negligible compared to the volume of the container
- The time of a collision is negligible compared to the time between collisions
- There are no forces of attraction or repulsion between the molecules
- The molecules are in continuous random motion
- The number of molecules of gas in a container is very large, therefore the average behaviour (eg. speed) is usually considered
Root-Mean-Square Speed
- The pressure of an ideal gas equation includes the mean square speed of the particles:
<c2>
- Where
- c = average speed of the gas particles
- <c2> has the units m2 s-2
- Since particles travel in all directions in 3D space and velocity is a vector, some particles will have a negative direction and others a positive direction
- When there are a large number of particles, the total positive and negative velocity values will cancel out, giving a net zero value overall
- In order to find the pressure of the gas, the velocities must be squared meaning that all the values end up positive
- To calculate the average speed of the particles in a gas, take the square root of the mean square speed:
![]()
- cr.m.s is known as the root-mean-square speed and still has the units of m s-1
Worked Example
An ideal gas has a density of 4.5 kg m-3 at a pressure of 9.3 × 105 Pa and a temperature of 504 K.
Determine the root-mean-square (r.m.s.) speed of the gas atoms at 504 K.
Step 1: Write out the equation for the pressure of an ideal gas with density
![]()
Step 2: Rearrange for mean square speed
![]()
Step 3: Substitute in values
![]()
Step 4: To find the r.m.s value, take the square root of the mean square speed
![]()
Step 5: Write the answer to the correct significant figures and include units
crms = 790 ms−1 (2 sig figs)
Deriving the Equation for Kinetic Theory
- When molecules rebound from a wall in a container, the change in momentum gives rise to a force exerted by the particle on the wall
- Many molecules moving in random motion exert forces on the walls which create an average overall pressure, since pressure is the force per unit area
The Situation for the Derivation
- Picture a single molecule in a cube-shaped box with sides of equal length l
- The molecule has a mass m and moves with speed c, parallel to one side of the box
- It collides at regular intervals with the ends of the box, exerting a force and contributing to the pressure of the gas
- By calculating the pressure this one molecule exerts on one end of the box, the total pressure produced by all the molecules can be deduced
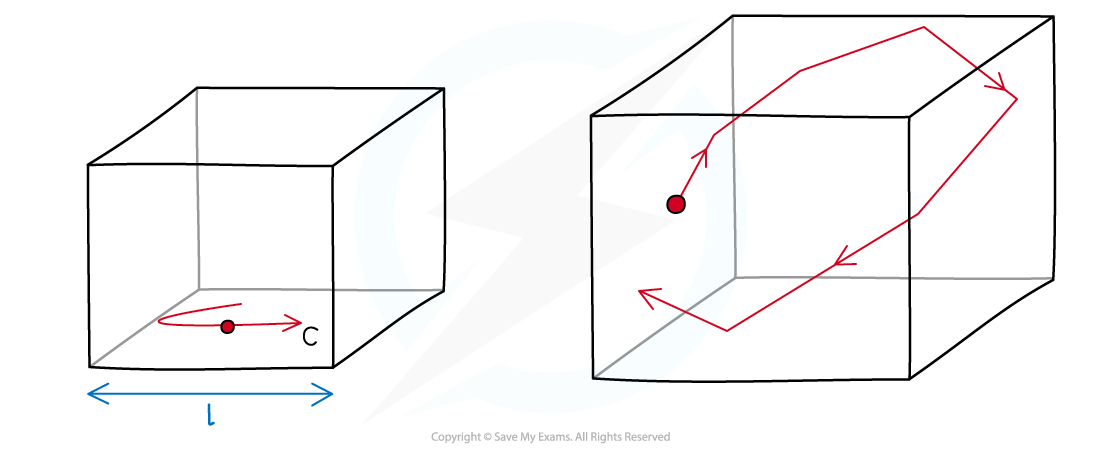
A single molecule in a box collides with the walls and exerts a pressure
Five-Step Derivation
Step 1: Find the change in momentum as a single molecule hits a wall perpendicularly
- One assumption of the kinetic theory is that molecules rebound elastically
- This means there is no kinetic energy lost in the collision
- If they rebound in the opposite direction to their initial velocity, their final velocity is -c
- The change in momentum is therefore:
![]()
Step 2: Calculate the number of collisions per second by the molecule on a wall
- The time between collisions of the molecule travelling to one wall and back is calculated by travelling a distance of 2l with speed c:

- Note: c is not taken as the speed of light in this scenario
Step 3: Find the change in momentum per second
- The force the molecule exerts on one wall is found using Newton’s second law of motion:

- The change in momentum is +2mc since the force on the molecule from the wall is in the opposite direction to its change in momentum
Step 4: Calculate the total pressure from N molecules
- The area of one wall is l2
- The pressure is defined using the force and area:
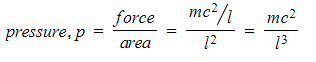
- This is the pressure exerted from one molecule
- To account for the large number of N molecules, the pressure can now be written as:
![]()
- Each molecule has a different velocity and they all contribute to the pressure
- The mean squared speed of c2 is written with left and right-angled brackets <c2>
- The pressure is now defined as:
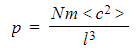
Step 5: Consider the effect of the molecule moving in 3D space
- The pressure equation still assumes all the molecules are travelling in the same direction and colliding with the same pair of opposite faces of the cube
- In reality, all molecules will be moving in three dimensions equally
- Splitting the velocity into its components cx, cy and cz to denote the amount in the x, y and z directions, c2 can be defined using pythagoras’ theorem in 3D:
![]()
- Since there is nothing special about any particular direction, it can be determined that:
![]()
- Therefore, <cx2> can be defined as:
![]()
- The box is a cube and all the sides are of length l
- This means l3 is equal to the volume of the cube, V
- Substituting the new values for <c2> and l3 back into the pressure equation obtains the final equation:
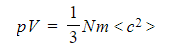
- This is known as the Kinetic Theory of Gases equation:
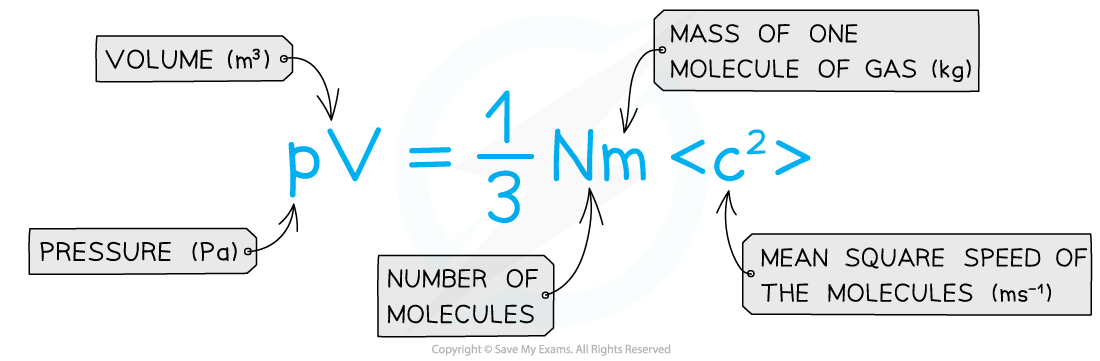
- It can also be written using the density ρ of the gas:
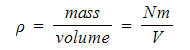
- Rearranging the pressure equation for p and substituting the density ρ:
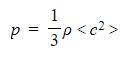
Exam Tip
Make sure to revise and understand each step for the whole of the derivation, as you may be asked to derive all, or part, of the equation in an exam question.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









