- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Physics:复习笔记9.1 Specific Heat Capacity & Latent Heat
Specific Heat Capacity
- Specific heat capacity is defined as:
the energy required to raise the temperature of one kilogram of a substance by one kelvin
- The increase in temperature of an object depends on:
- The amount of heat energy transferred
- The mass of the object
- The specific heat capacity of the material from which the object is made
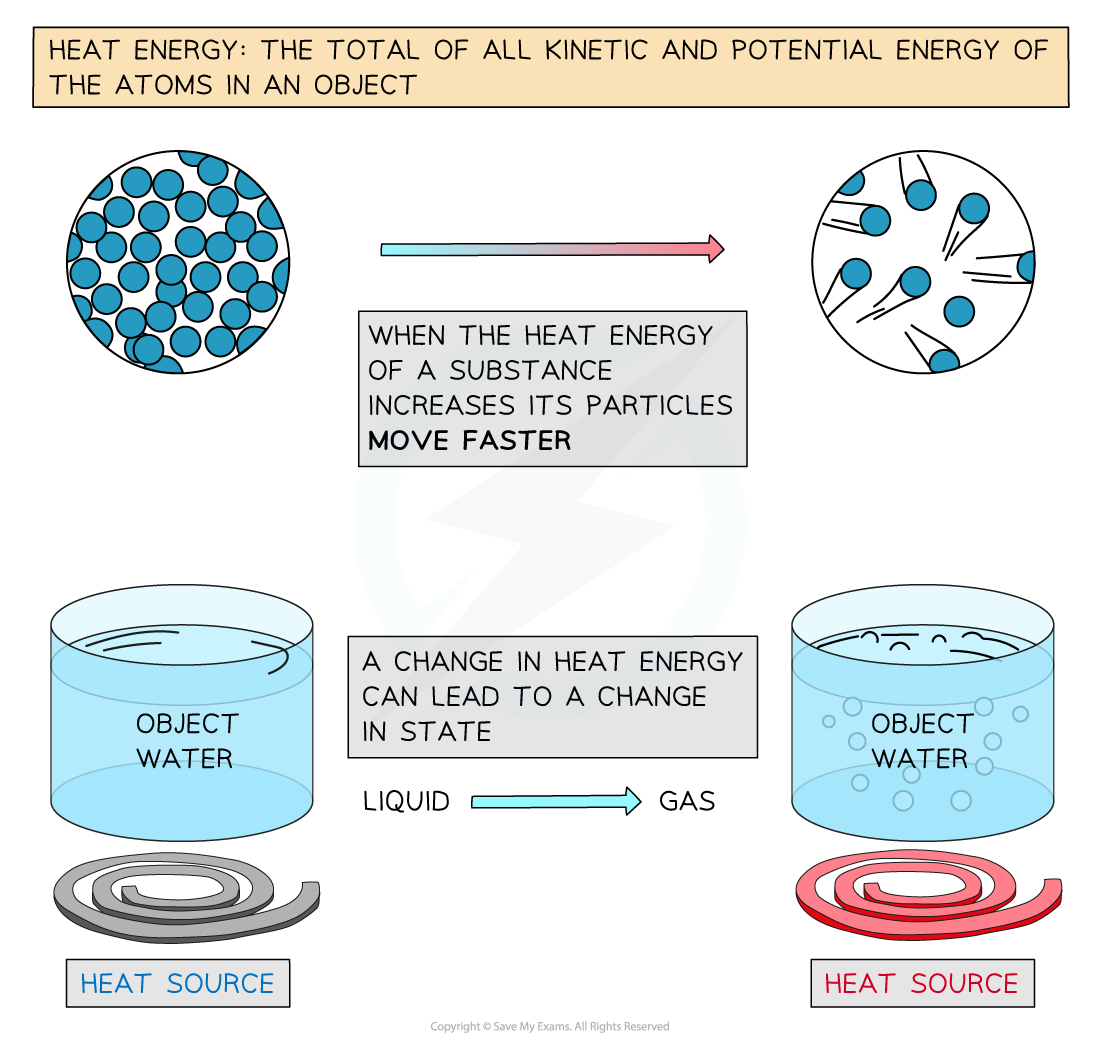
Explanation of Heat Energy
- The energy input is in the form of heat energy
- The amount of heat energy needed is given by the equation:
ΔE = mcΔθ
- Where:
- ΔE = change in heat energy, in joules (J)
- m = mass, in kilograms (kg)
- c = specific heat capacity, in joules per kilogram per degree Kelvin (J/kg K or J/kg °C)
- Δθ = change in temperature, in Kelvin or Celsius
- (The symbol Δ in Maths is used to denote a change in value)

Examples of heat energy
- The thermodynamic Kelvin temperature scale is defined as:
An absolute temperature scale where each degree is the same size as those on the Celsius scale
- Different materials:
- Have different specific heat capacities because of their molecular structure
- Have different rises in temperature for the same change in heat energy
- Specific heat capacity is mainly used for liquids and solids
- Good electrical conductors, such as copper and lead, are excellent conductors of heat due to their low specific heat capacity
- On the other hand, water has a very high specific heat capacity, making it ideal for heating homes as the water remains hot in a radiator for a long time
- The specific heat capacity of different substances determines how useful they would be for a specific purpose eg. choosing the best material for kitchen appliances
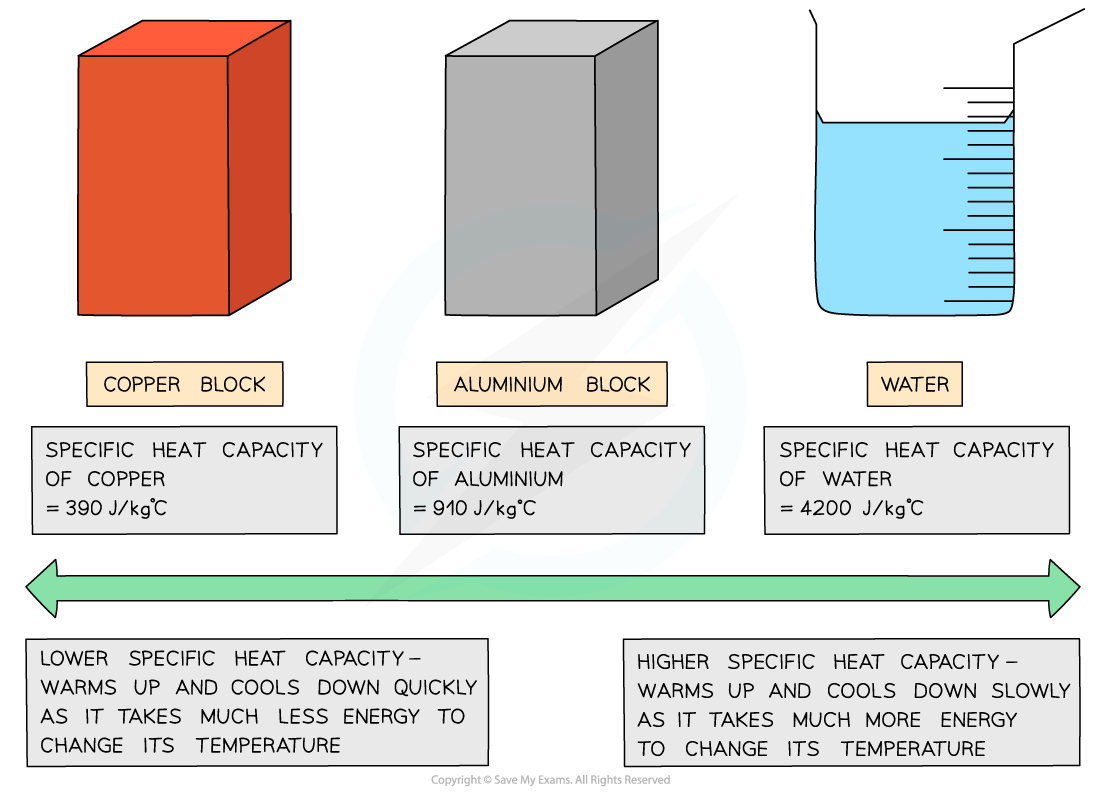
Low v high specific heat capacity
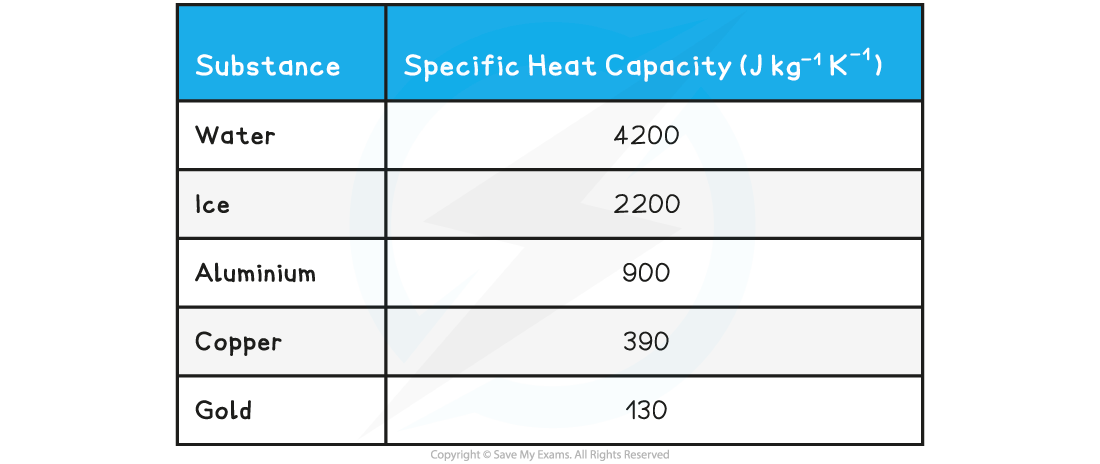
- The specific heat capacity of some substances are given in the table below as examples:
Table of values of specific heat capacity for various substances
Worked Example
Water of mass 0.48 kg is increased in temperature by 0.7 K. The specific heat capacity of water is 4200 J kg-1 K-1. Calculate the amount of energy transferred to the water.
Step 1: Write down the known quantities
-
- Mass, m = 0.48 kg
- Change in temperature, Δθ = 0.7 K
- Specific heat capacity, c = 4200 J kg-1 K-1
Step 2: Write down the relevant equation
ΔE = mcΔθ
Step 3: Calculate the energy transferred by substituting in the values
ΔE = (0.48) × (4200) × (0.7) = 1411.2
Step 4: Round the answer to 2 significant figures
ΔE = 1400 J
Exam Tip
You will always be given the specific heat capacity of a substance, so you do not need to memorise any values. Make sure that Δθ is the change in temperature, therefore, it can be in K or °C.
Specific Latent Heat
- Energy is required to change the state of substance
- Examples of changes of state are:
- Melting = solid to liquid
- Evaporation/vaporisation/boiling = liquid to gas
- Sublimation = solid to gas
- Freezing = liquid to solid
- Condensation = gas to liquid
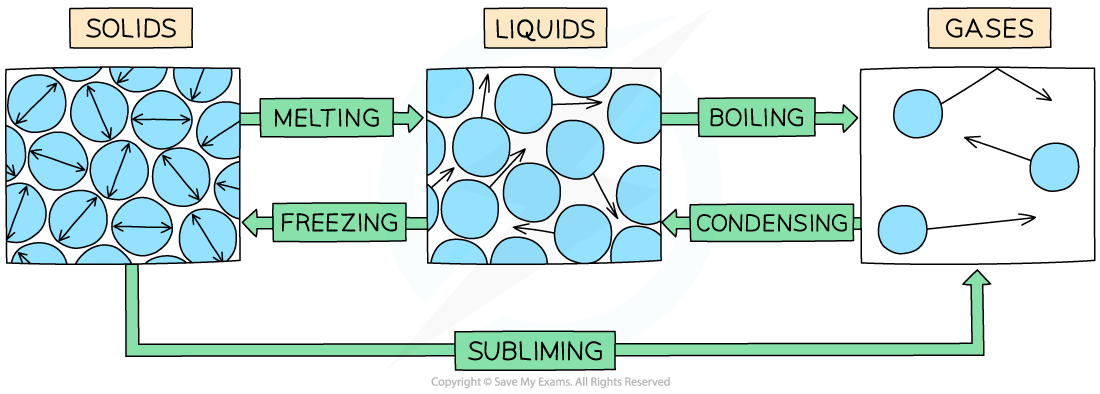
The example of changes of state between solids, liquids and gases
- When a substance changes state, there is no temperature change
- The energy supplied to change the state is called the latent heat and is defined as:
The thermal energy required to change the state of one kilogram of a substance without any change of temperature
- There are two types of latent heat:
- Specific latent heat of fusion (melting)
- Specific latent heat of vaporisation (boiling)
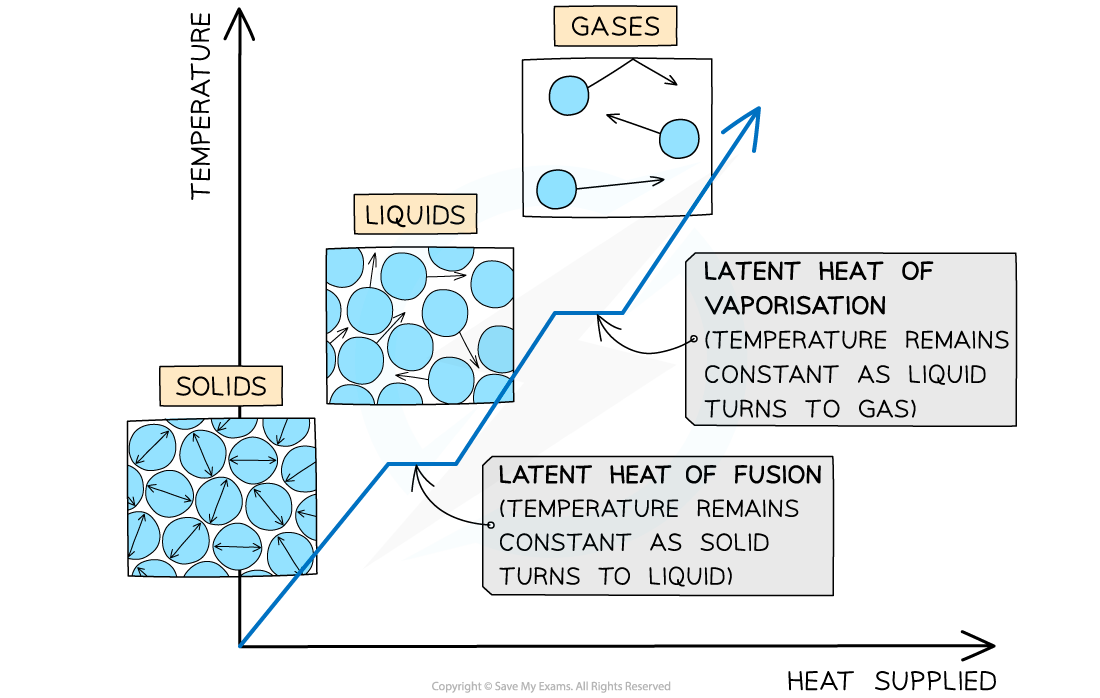
The changes of state with heat supplied against temperature. There is no change in temperature during changes of state
- The specific latent heat of fusion is defined as:
The thermal energy required to convert one kilogram of solid to liquid with no change in temperature
-
- This is used when melting a solid or freezing a liquid
- The specific latent heat of vaporisation is defined as:
The thermal energy required to convert one kilogram of liquid to gas with no change in temperature
-
- This is used when vaporising a liquid or condensing a gas
Calculating Specific Latent Heat
- The amount of energy ΔE required to melt or vaporise a mass of m with latent heat L is:
ΔE = LΔm
- Where:
- ΔE = amount of heat energy to change the state (J)
- L = latent heat of fusion or vaporisation (J kg-1)
- Δm = change in mass of the substance changing state (kg)
- The values of latent heat for water are:
- Specific latent heat of fusion = 330 kJ kg-1
- Specific latent heat of vaporisation = 2.26 MJ kg-1
- Therefore, evaporating 1 kg of water requires roughly seven times more energy than melting the same amount of ice to form water
- The reason for this is to do with intermolecular forces:
- When ice melts: energy is required to just increase the molecular separation until molecules can flow freely over each other
- When water boils: energy is required to completely separate the molecules until there are no longer forces of attraction between them, hence this requires much more energy
Worked Example
The energy needed to boil a mass of 530 g of a liquid is 0.6 MJ. Calculate the specific latent heat of the liquid and state whether it is the latent heat of vaporisation or fusion.
Step 1: Substitute in the values
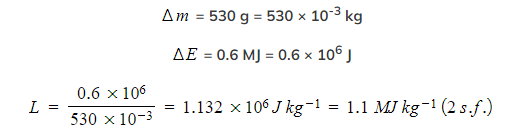
L is the latent heat of vaporisation because the change in state is from liquid to gas (boiling)
Exam Tip
Use these reminders to help you remember which type of latent heat is being referred to:
- Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
- Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








