- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Physics:复习笔记7.3 Electric Force between Two Charges
Electric Force between Two Charges
- All charged particles produce an electric field around them
- This field exerts a force on any other charged particle within range
- The electrostatic force between two charges is defined by Coulomb’s Law
- Recall that the charge of a uniform spherical conductor can be considered as a point charge at its centre
- Coulomb’s Law states that:
The electrostatic force between two point charges is proportional to the product of the charges and inversely proportional to the square of their separation
- The force FE between two charges as expressed by Coulomb's Law is given by the equation:
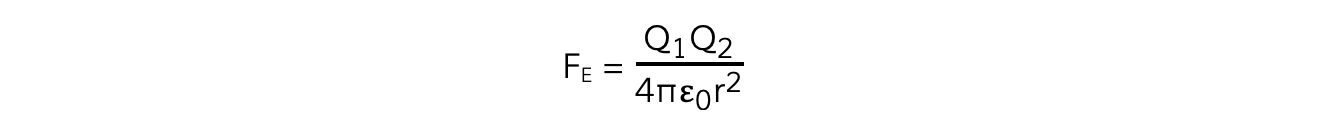

The electrostatic force between two charges is defined by Coulomb’s Law
- Where:
- FE = electrostatic force between two charges (N)
- Q1 and Q2 = two point charges (C)
- ε0 = permittivity of free space
- r = distance between the centre of the charges (m)
- The 1/r2 relation is called the inverse square law
- This means that when the separation of two charges doubles, the electrostatic force between them reduces to (½)2 = ¼ of its original size
- ε0 is a physical constant used to show the capability of a vacuum to permit electric fields
- If Q1 and Q2 are oppositely charged, then the electrostatic force FE is negative
- This can be interpreted as an attractive force between Q1 and Q2
- If Q1 and Q2 are the same charge, then the electrostatic force FE is positive
- This can be interpreted as a repulsive force between Q1 and Q2
Worked Example
An alpha particle is situated 2.0 mm away from a gold nucleus in a vacuum. Assuming they are point charges, calculate the magnitude of the force acting on each of the charges.
Atomic number of helium = 2
Atomic number of gold = 79
Charge of an electron = 1.60 × 10-19 C
Step 1: Write down the known quantities
-
- Distance, r = 2.0 mm = 2.0 × 10-3 m
The charge of one proton = +1.60 × 10-19 C
An alpha particle (helium nucleus) has 2 protons
-
- Charge of alpha particle, Q1 = 2 × 1.60 × 10-19 = +3.2 × 10-19 C
The gold nucleus has 79 protons
-
- Charge of gold nucleus, Q2 = 79 × 1.60 × 10-19 = +1.264 × 10-17 C
Step 2: The electrostatic force between two point charges is given by Coulomb’s Law
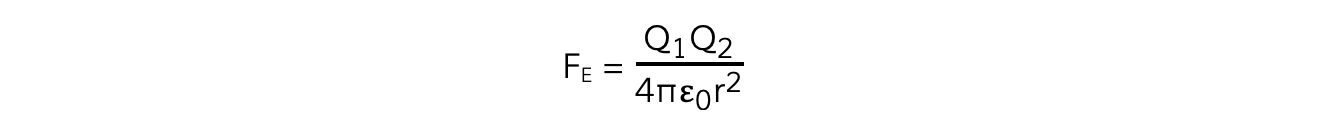
Step 3: Substitute values into Coulomb's Law
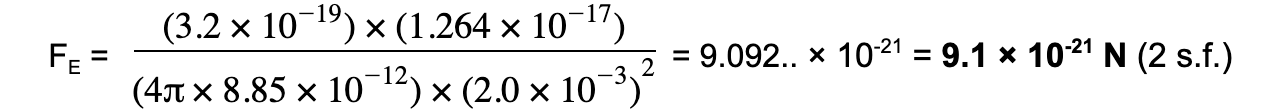
Exam Tip
Remember to always square the distance, r between the charges!
Always look out for unit prefixes when substituting values into an equation. Check whether the charge has been converted into C instead of nC or µC, or the distance in mm to m to get a force F of newtons, N.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









