- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.6.1 Deducing Organic Structures
Deducing Organic Structures
- You will be expected to deduce the empirical, molecular and structural formulae of compounds from data such as:
- Combustion analysis
- Elemental percentage composition - also known as calculating empirical formulae
- Characteristic reactions of functional groups - this can include test-tube reactions as well as interconversions
- Infrared (IR) spectra
- Mass spectra (MS)
- Nuclear magnetic resonance (NMR) spectra
- The normal progression for the analysis of a compound is:
- Find the empirical formula
- Determine the molecular formula
- Identify the functional groups / structural components present
- Deduce the overall structure
Combustion analysis
- Sometimes combustion analysis is performed on an unknown compound to determine the elemental percentage composition
- The elemental percentage composition is then used to calculate the empirical formula
- In combustion analysis, a known mass of the compound is burned in an excess of dry oxygen
- The mass of carbon dioxide and water are then used to determine the percentage content of carbon, hydrogen and oxygen in the compound
- This can also be applied to include sulfur and nitrogen, although you are not expected to do this, at this level
- To convert combustion analysis data to elemental percentage composition:
- Calculate the mass of carbon in the sample from the combustion analysis results
- Calculate the percentage of carbon in the sample
- Calculate the mass of hydrogen in the sample from the combustion analysis results
- Calculate the percentage of hydrogen in the sample
- Use the percentage of carbon and hydrogen to deduce the percentage of oxygen in the sample
Worked Example
Combustion analysis was performed on 2.90 g of an unknown carbohydrate, A.
6.60 g of carbon dioxide and 2.70 g of water were produced.
Calculate the carbohydrates percentage composition.
Answer
Step 1: Calculate the mass of carbon in the sample
-
- Mass of carbon =
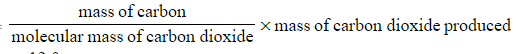
- Mass of carbon
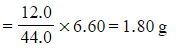
- Mass of carbon =
Step 2: Calculate the percentage of carbon in the sample
-
- Percentage carbon =
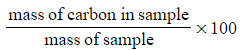
- Percentage carbon=
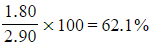
- Percentage carbon =
Step 3: Calculate the mass of hydrogen in the sample
-
- Mass of hydrogen =
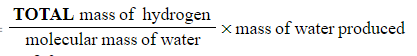
- Mass of hydrogen
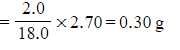
- Mass of hydrogen =
Step 4: Calculate the percentage of hydrogen in the sample
-
- Percentage hydrogen =
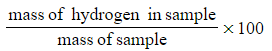
- Percentage hydrogen
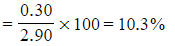
- Percentage hydrogen =
Step 5: Calculate the percentage of oxygen in the sample
-
- Percentage oxygen = 100 - percentage of carbon - percentage of hydrogen
- Percentage oxygen = 100 - 62.1 - 10.3 = 27.6%
- Therefore, the percentage composition of the carbohydrate is 62.1% carbon, 10.3% hydrogen and 27.6% oxygen
Exam Tip
Don't forget to use 2.0 g for the mass of hydrogen as there are two hydrogen atoms in a water molecule
Worked Example
Calculate the empirical formula for the unknown carbohydrate, A
Answer
-
- The percentage composition of the unknown carbohydrate, A, is 62.1% carbon, 10.3% hydrogen and 27.6% oxygen
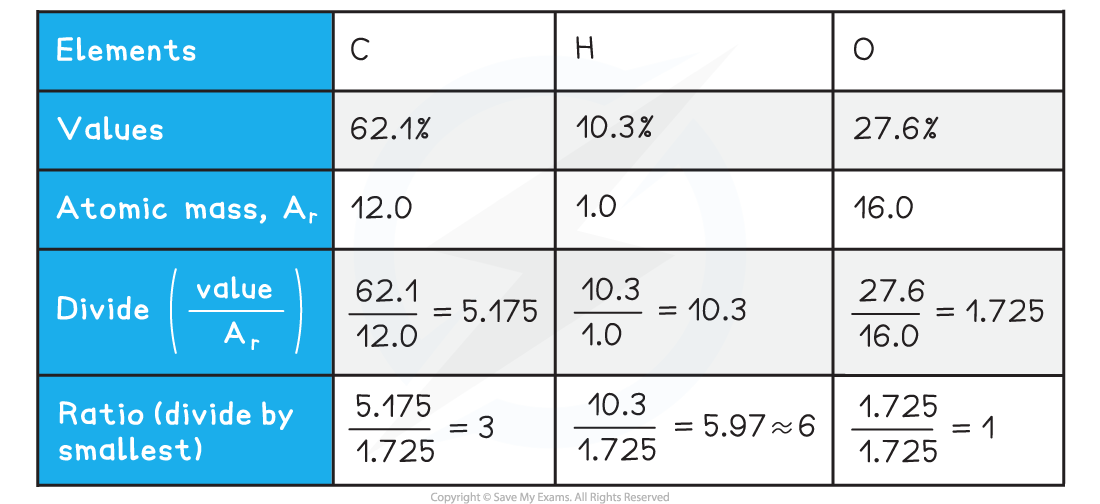
-
- Empirical formula = C3H6O
Characteristic reactions of functional groups
- For deducing structures, these will typically be the test-tube reactions, including (but not limited to):
- Bromine: C=C bond
- Acidified potassium dichromate(VI) solution: primary and secondary alcohols
- Fehling's or Tollens': aldehydes and ketones
- 2,4-dinitrophenylhydrazine (2,4-DNPH): C=O bond
- Sodium carbonate: carboxylic acids
- Iodoform: Methyl groups next to C=O
Spectral analysis
- These will include:
- Infrared spectroscopy: to identify functional groups and certain bond types
- Mass spectrometry: to identify molecular formula and fragments
- Carbon-13 (13C) nuclear magnetic resonance: to identify compound structure
- Proton (1H) nuclear magnetic resonance: to identify compound structure
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









