- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.5.7 Amino Acids & Proteins
Properties of 2-Amino Acids
Acid / base properties of amino acids
- Amino acids will undergo most reactions of amines and carboxylic acids including acid-base reactions of:
- Amines with acids
- Carboxylic acids with bases
- However, they can also interact intramolecularly (within themselves) to form a zwitterion
- A zwitterion is an ion with both a positive (-NH3+) and a negative (-COO-) charge
- Because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids
- Amino acids are therefore soluble crystalline solids

An amino acid molecule can interact within itself to form a zwitterion
Isoelectric point
- A solution of amino acids in water will exist as zwitterions with both acidic and basic properties
- They act as buffer solutions as they resist any changes in pH when small amounts of acids or alkali are added
- If an acid is added (and thus the pH is lowered):
- The -COO- part of the zwitterion will accept an H+ ion to reform the -COOH group
- This causes the zwitterion to become a positively charged ion
- If a base is added (and thus the pH is raised):
- The -NH3+ part of the zwitterion will donate an H+ ion to reform the -NH2 group
- This causes the zwitterion to become a negatively charged ion
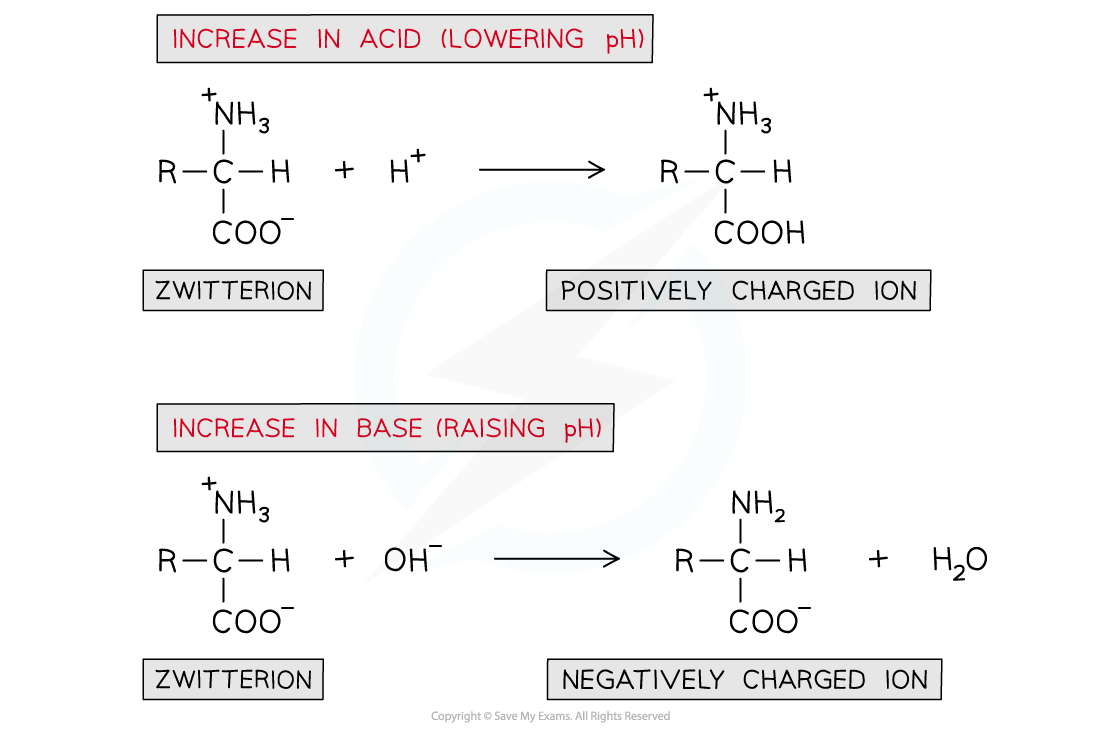
A solution of amino acids can act as a buffer solution by resisting any small changes in pH
- The pH can be slightly adjusted to reach a point at which neither the negatively charged or positively charged ions dominate and the amino acid exists as a neutral zwitterion
- This is called the isoelectric point of the amino acid
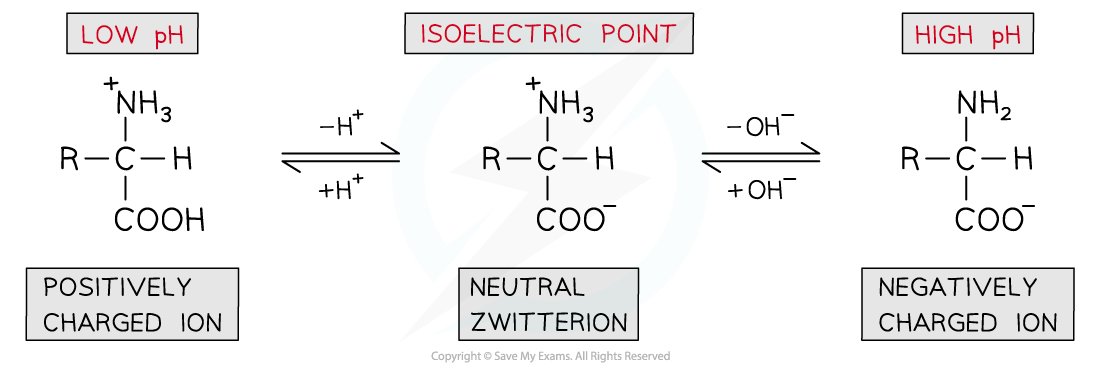
The isoelectric point of amino acids is the pH at which the amino acid exists as a neutral zwitterion
Reactions of the amine group
- The amine group is basic and reacts with acids to make salts
- For example, a general amino acid reacts with hydrochloric acid to form the ammonium salt:
H2NCHRCOOH + HCl ⇌ H3N+CHRCOOH + Cl-
Reactions of the carboxylic acid group
Reaction with aqueous alkalis
- An amino acid reacts with aqueous alkali such as sodium or potassium hydroxide to form a salt and water
- For example, a general amino acid reacts with sodium hydroxide to form a sodium salt:
H2NCHRCOOH + NaOH ⇌ H2NCHRCOO- Na+ + H2O
Esterification with alcohols
- Amino acids, like carboxylic acids, can be esterified by heating with alcohol in the presence of concentrated sulfuric acid
- The carboxylic acid group is esterified whilst the basic amine group is protonated due to the acidic conditions:
H2NCHRCOOH + C2H5OH + H+ ⇌ H3N+CHRCOOC2H5 + H2O
Optical activity
- Almost all 2-amino acids contain a chiral centre (the C of the CH group), and so are optically active
- The only exception is glycine, which has a CH2 group instead
- Aqueous solutions of the enantiomers rotate the plane of polarisation of plane-polarised light
- Dextrorotatory (+)
- Laevorotatory (-)
- If an amino acid is synthesised in the lab, a racemic mixture is formed
The Peptide Bond
- Each amino acid contains an amine (-NH2) and carboxylic acid (-COOH) group
- The new amide bond between two amino acids is also called a peptide link or peptide bond
-
- The -NH2 group of one amino acid can react with the -COOH group of another amino acid in a condensation reaction to form a dipeptide
- Since this is a condensation reaction, a small molecule (in this case H2O) is eliminated
- The dipeptide still contains an -NH2 and -COOH group at each end of the molecule which can again participate in a condensation reaction to form a tripeptide
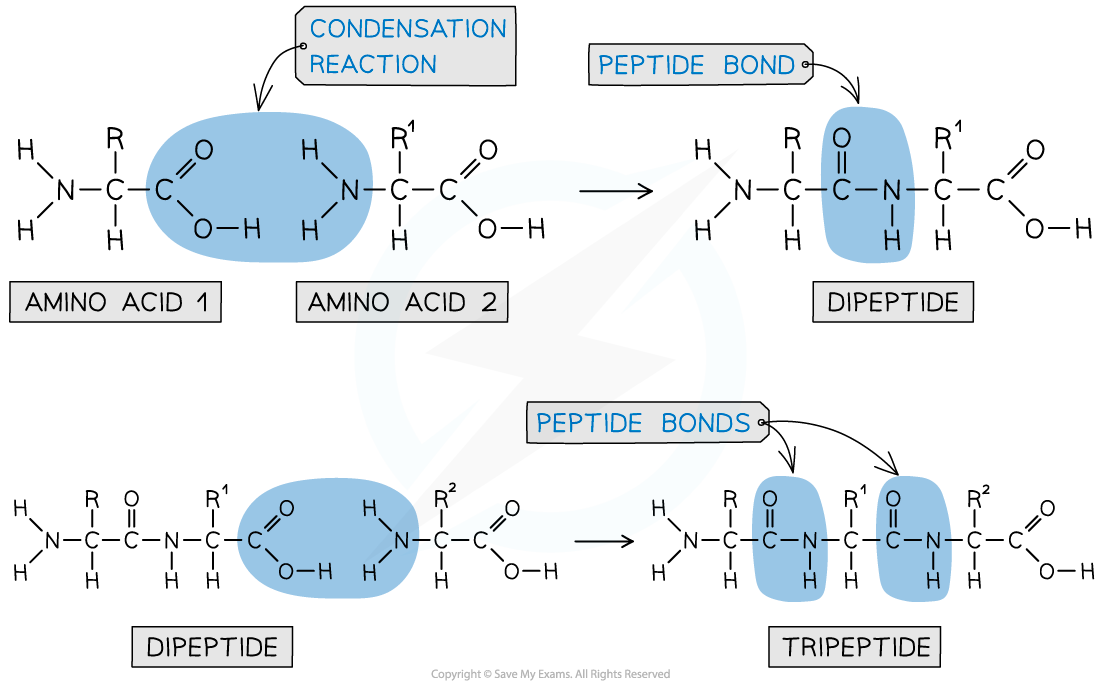
A peptide bond is an amide bond between two amino acids
- A polypeptide is formed when many amino acids join together to form a long chain of molecules
Hydrolysing Proteins
- The polypeptide chains in a protein can be broken down into their individual amino acids by prolonged heating with concentrated hydrochloric acid.
- This breaks the peptide bonds between amino acids
- Due to the acidic environment the amino acids formed will have their NH2 groups protonated as +NH3 groups
Using Chromatography
- The amino acids produced by hydrolysis can be identified using simple chromatography
- Using a suitable solvent, the individual amino acids will rise to different heights on the chromatography paper
- As the amino acids are colourless, the chromatogram is sprayed with a developing agent so that the amino acid positions can be seen
- Once the positions of the amino acids have been established, their Rf values can be calculated
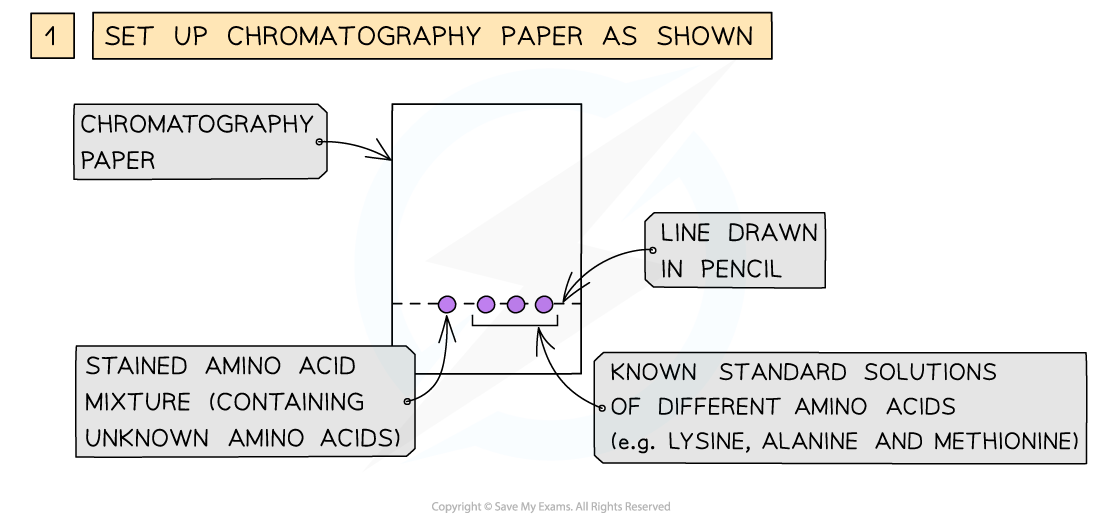
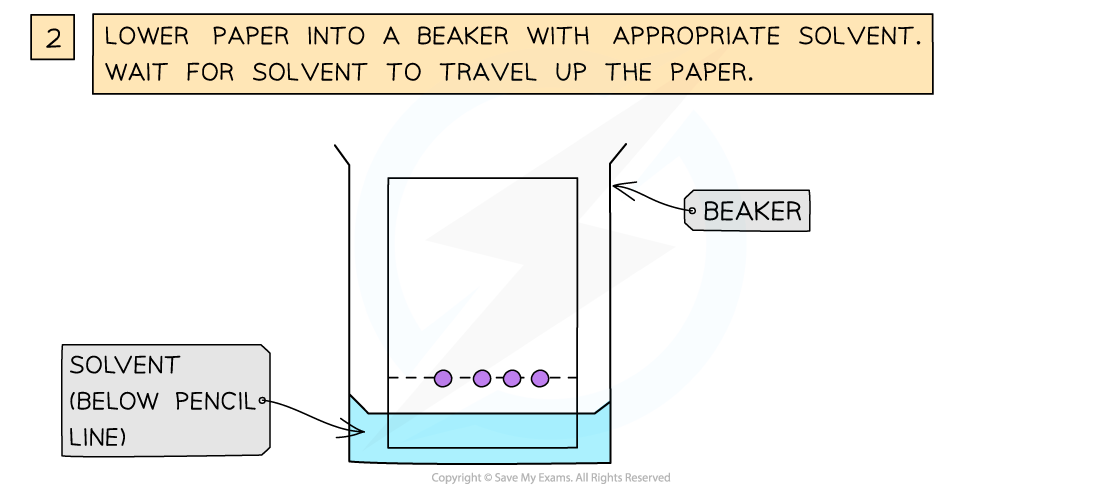
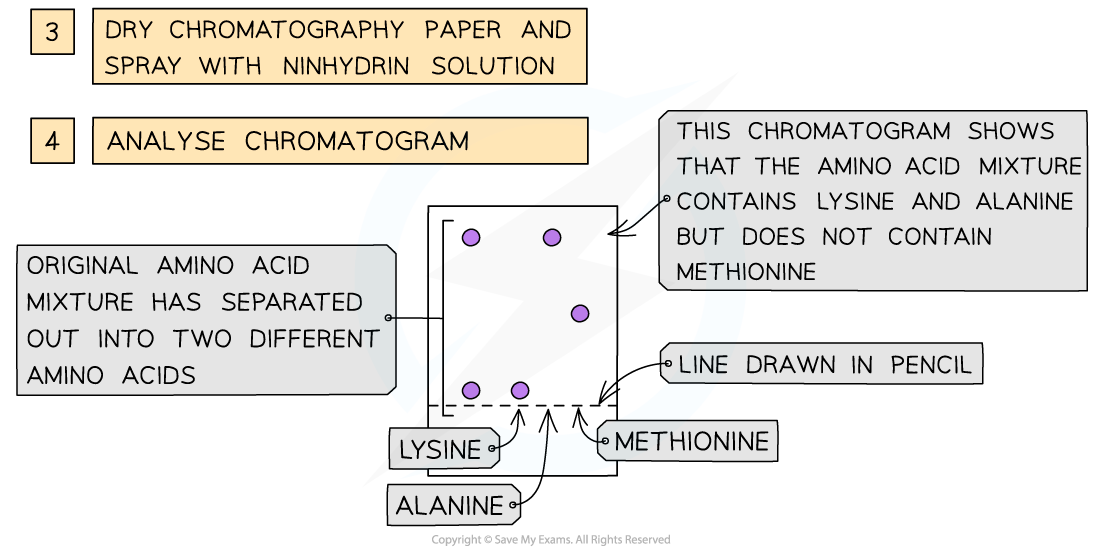
How chromatography can be used to separate a mixture of amino acids and identify the individual components
Exam Tip
Remember that separating amino acids using chromatography depends on the relative solubilities of the amino acids in the mobile and stationary phases.
It does not depend on the size of the amino acids.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








