- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.5.3 Amine Basicity
Amine Basicity
- The nitrogen atom in ammonia and amine molecules can accept a proton (H+ ion)
- They can therefore act as Bronsted-Lowry bases in aqueous solutions by donating its lone pair of electrons to a proton and form a dative bond
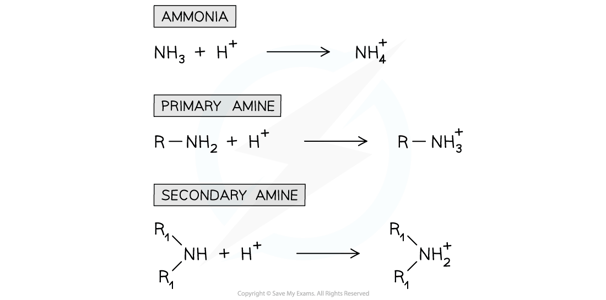
The nitrogen atom in ammonia and amines can donate its lone pair of electrons to form a bond with a proton and therefore act as a base
- The strength of amines depends on the ability of the lone pair of electrons on the nitrogen atom to accept a proton and form a dative covalent bond
- The more readily a proton is attracted, the stronger the base is
- Factors that may affect the basicity of amines include:
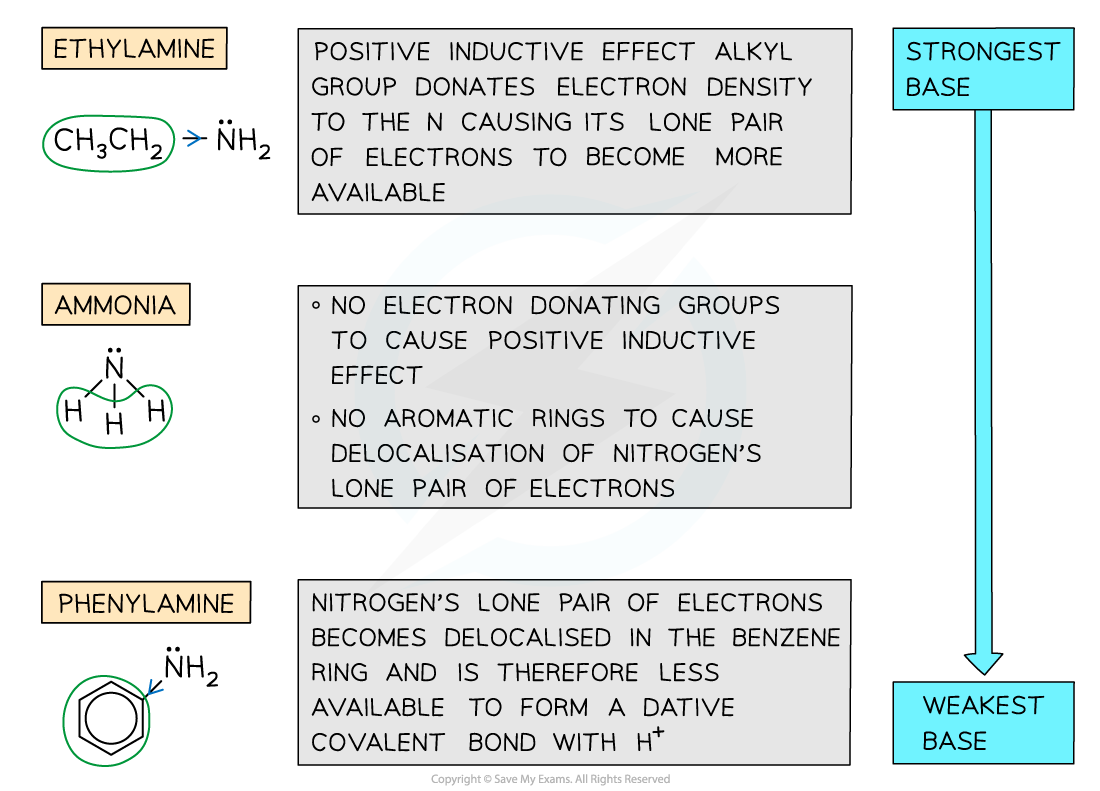
- Positive inductive effect - Some groups such as alkyl groups donate electron density to the nitrogen atom causing the lone pair of electrons to become more available and therefore increasing the amine’s basicity
- Delocalisation - The presence of aromatic rings such as the benzene ring causes the lone pair of electrons on the nitrogen atom to be delocalised into the benzene ring
- The lone pair becomes less available to form a dative covalent bond with ammonia and hence decreases the amine’s basicity
- Primary aliphatic amines are stronger bases than ammonia as the alkyl groups are electron releasing and push electrons towards the nitrogen atom and so make it a stronger base
- Secondary amines are stronger bases than primary amines because they have more alkyl groups that are substituted onto the nitrogen atom in place of hydrogen atoms
- Therefore more electron density is pushed onto the nitrogen atom (as the inductive effect of alkyl groups is greater than that of hydrogen atoms)
- Therefore more electron density is pushed onto the nitrogen atom (as the inductive effect of alkyl groups is greater than that of hydrogen atoms)
Base strength of aromatic amines
- Primary aromatic amines such as phenylamine do not form basic solutions because the lone pair of electrons on the nitrogen delocalise with the ring of electrons in the benzene ring
- This means the nitrogen is less able to accept protons
- Ethylamine (which has an electron-donating ethyl group) is more basic than phenylamine (which has an electron-withdrawing benzene ring)

Ethylamine is more basic than phenylamine due to electron donating ethyl group which increases electron density on the nitrogen and makes it more attractive to protons
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








