- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.5.2 Primary Aliphatic Amines
Reactions of Primary Aliphatic Amines
Reactions with water
- The first few members of the homologous series of primary aliphatic amines are miscible with water
- However as the hydrocarbon part of the molecule becomes longer, the solubility decreases
- Phenylamine is only slightly soluble in water
- They dissolve in water as they are able to form hydrogen bonds with water molecules
- Amines also react slightly with water to form alkaline solutions
CH3NH2 + H2O ⇌ CH3NH3+ + OH-
Reactions with acids
- Amines react with strong acids to form ionic ammonium salts
CH3NH2 (aq) + HCl (aq) → CH3NH3+Cl- (aq)
Methylamine methylammonium chloride
- Addition of NaOH to an ammonium salt will convert it back to the amine
- These ionic salts will be solid crystals, if the water is evaporated, because of the strong ionic interactions
- The ionic salts formed in this reaction means that the compounds are soluble in the acid
- e.g. Phenylamine is not very soluble in water but phenylammonium chloride is soluble
Reactions with ethanoyl chloride
- This reaction type is addition-elimination reaction meaning two molecules join together, and then a small molecule is eliminated - in these examples, hydrogen chloride
- You do not need to know the mechanism of these reactions
- The organic product contains a new functional group - amide - in which a carbonyl group is next to an NH group
- The equation for the reaction of butylamine with ethanoyl chloride is
CH3COCl + CH3CH2CH2CH2NH2 → CH3CONHCH2CH2CH2CH3 + HCl
Reaction with halogenoalkanes
- Again you do not need to know the mechanism for these reactions
- The electron-deficient carbon atom in the halogenoalkane and the electron-rich atom nitrogen atom in the amine causes these two species to react together
- The general formula for this reaction would be
R'NH2 + R"X → R'NHR" + HX
- Where R' is the alkyl group in the amine and R" is the alkyl group in the halogenoalkane
- This reaction is an example of a substitution reaction
- The organic product is a secondary amine and the inorganic product is a hydrogen halide, often hydrogen chloride
- As an example, the equation for the reaction of butylamine and chloroethane is
CH3CH2CH2CH2NH2 + CH3CH2Cl → CH3CH2CH2CH2NHCH2CH3 + HCl
- The organic product contains an electron-rich nitrogen atom, so can also react with chloroethane
CH3CH2CH2CH2NHCH2CH3 + CH3CH2Cl → CH3CH2CH2CH2N(CH2CH3)2 + HCl
- The organic product of this reaction is a tertiary amine
- The organic product also contains an electron-rich nitrogen atom, so can also react with chloroethane
CH3CH2CH2CH2N(CH2CH3)2 + CH3CH2Cl → CH3CH2CH2CH2N+(CH2CH3)3Cl-
- In this reaction HCl is not formed because this would require the loss of H from the nitrogen from the organic reactant, which the tertiary amine doesn't have
- The product is an ionic compound related to ammonium chloride except that all the hydrogens in the ammonium ion have been replaced by alkyl groups
- This is known as a quaternary ammonium salt
Reactions with copper(II) ions
- Ammonia can act as a lone pair donor in its reactions with transition metal ions
- For example the overall equation for the reaction of ammonia with hexaaquacopper(II) ions is
[Cu(H2O)6]2+ + 4NH3→ [Cu(NH3)4(H2O)2]2+ + 4H2O
- Amines also have a lone pair of electrons on the nitrogen, so can take part in similar reactions
- The observations are the same as with ammonia
- A blue precipitate forms
- With excess butylamine the precipitate dissolves to give a blue solution
- Formation of the pale blue precipitate
[Cu(H2O)6]2+ + 2CH3CH2CH2CH2NH2 → [Cu(H2O)4(OH)2] + 2CH3CH2CH2CH2NH3+
- Formation of the deep blue solution
[Cu(H2O)4(OH)2] + 4CH3CH2CH2CH2NH2 → [Cu(CH3CH2CH2CH2NH2)4(H2O)2]2+ + 2H2O +2OH-
Preparation of Primary Aliphatic Amines
Preparing Amines
- Primary amines can be prepared from different reactions including:
- The reaction of halogenoalkanes with ammonia
- The reduction of nitriles
Reaction of halogenoalkanes with ammonia
- This is a nucleophilic substitution reaction in which the nitrogen lone pair in ammonia acts as a nucleophile and replaces the halogen in the halogenoalkane
- When a halogenoalkane is reacted with excess, hot ethanolic ammonia under pressure a primary amine is formed

Formation of primary amine
Reduction of nitriles
- Nitriles contain a -CN functional group which can be reduced to an -NH2 group
- The nitrile vapour and hydrogen gas are passed over a nickel catalyst or LiAlH4 in dry ether can be used to form a primary amine
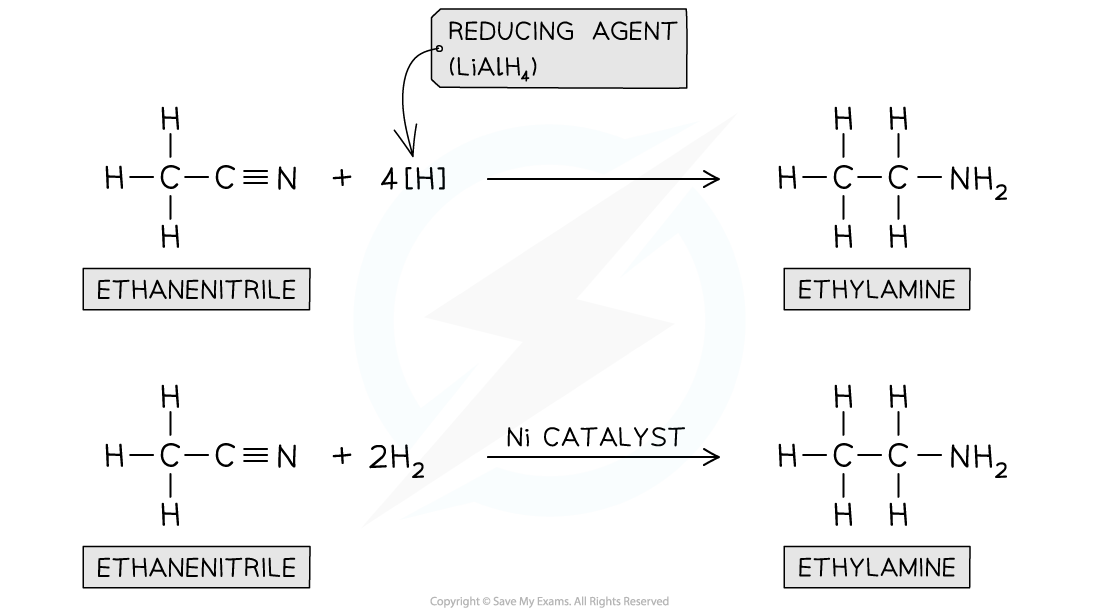
Nitriles can be reduced with LiAlH4 or H2 and Ni catalyst
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








