- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记7.3.1 Physical Properties of Carboxylic Acids
Physical Properties of Carboxylic Acids
Carboxylic acids
- Carboxylic acid is the name given to the family of compounds that contain the carboxyl functional group, -COOH
- The general formula of a carboxylic acid is CnH2n+1COOH which can be shortened to just RCOOH
- (In some countries, this family is also called alkanoic acid)
- The nomenclature of carboxylic acid follows the pattern alkan + oic acid, e.g. propanoic acid
- There is no need to use numbers in the name as the carboxyl group, COOH, is always on the number 1 carbon atom
Carboxylic Acids Examples Table
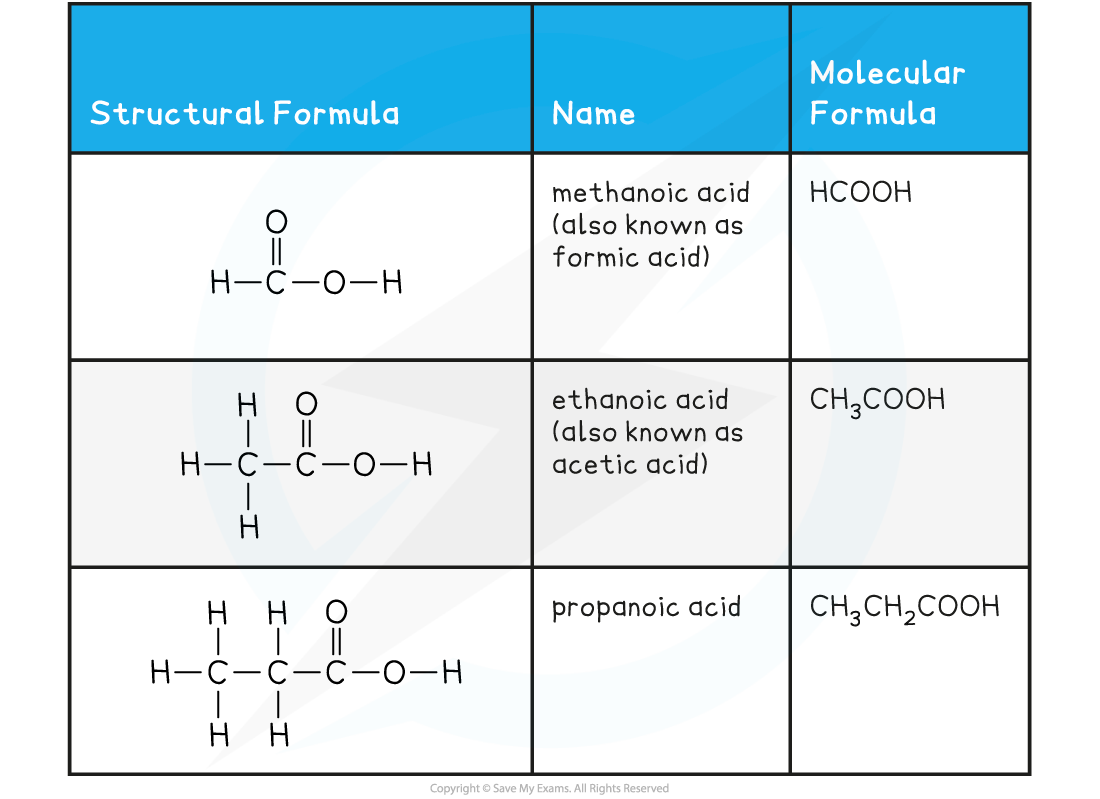
Physical properties of carboxylic acids
- Carboxylic acids contain two polarised groups
- C=O and O-H
- This means that the intermolecular forces that carboxylic acids experience are high and they will have relatively high melting points and boiling points
- The presence of the O-H bond means that they can exhibit hydrogen bonding
- Not only does this contribute to the high melting and boiling points, it also contributes to the solubility in water and other polar solvents of the shorter chained carboxylic acids
- However, solubility falls as the length of the hydrocarbon chain in the carboxylic acid increases
- The hydrocarbon chains are forcing their way between water molecules and so breaking hydrogen bonds between those water molecules
- Carboxylic acids with more than eight carbon atoms will be solids at room temperature and are very slightly soluble in cold water, but will be more soluble in hot water
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









