- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.3.1 Vanadium
Colours & Oxidation States
- Vanadium is a transition metal which has variable oxidation states
- The table below shows the important ones you need to be aware of
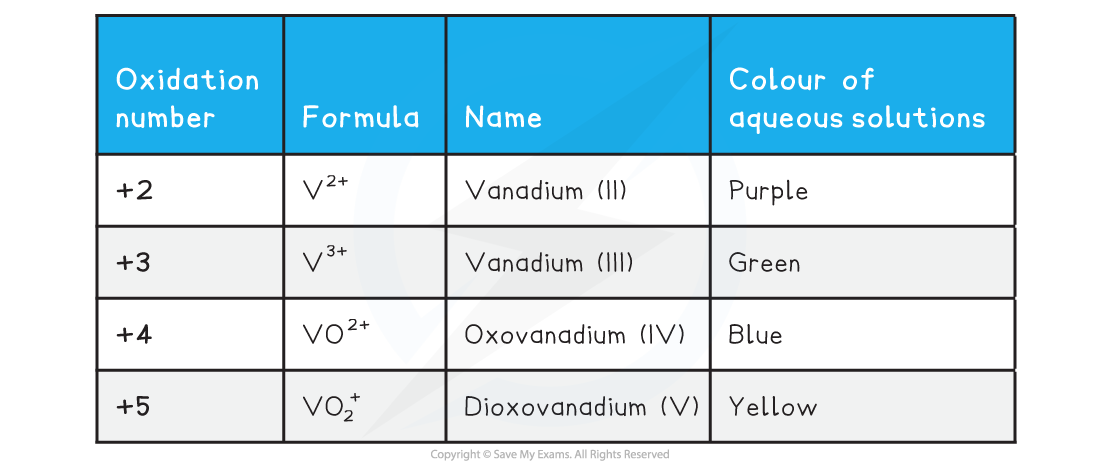
- Addition of zinc to the vanadium(V) in acidic solution will reduce the vanadium down through each successive oxidation state
- The colour would successively change from yellow to blue to green to violet
- The ion with the V at oxidation state +5 exists as a solid compound in the form of a VO3- ion
- Usually as NH4VO3 known as ammonium vanadate(V)
- It is a reasonably strong oxidising agent
- Addition of acid to the solid will turn into the yellow solution containing the VO2+ ion.
Interconversions of Vanadium Ions
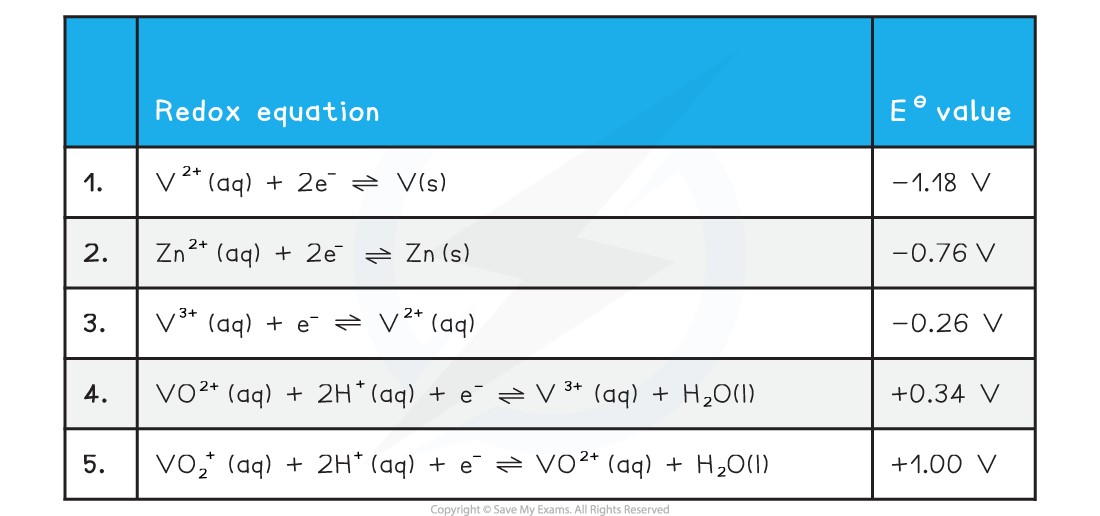
- For vanadium we need to consider the following standard electrode potential values
- We will use zinc as our chosen oxidising agent
- The half equations are arranged from high negative EΘ at the top to high positive EΘ at the bottom
- The best reducing agent is the top right species (V2+)
- The best oxidising agent is the bottom left species (VO2+)
Reduction from +5 to +4
- The two half equations we need to consider are 2 and 5
- Vanadium is reduced from an oxidation number of +5 to +4 in half equation 5
- The EΘ value for half equation 2 is more negative than the EΘ for half equation 5
- Zn is the best reducing agent
- VO2+ is the best oxidising agent
- We can obtain the overall equation by reversing half equation 2 and combining with equation 5
- When adding half equations remember to multiply them so each have the same number of electrons
2VO2+ (aq) + 4H+ (aq) + Zn (s) → 2VO2+ (aq) + Zn2+ (aq) + 2H2O (l)
Reduction from +4 to +3
- The two half equations we need to consider are 2 and 4
- Vanadium is reduced from an oxidation number of +4 to +3 in half equation 4
- The EΘ value for half equation 2 is more negative than the EΘ for half equation 5
- Zn is the best reducing agent
- VO2+ is the best oxidising agent
- We can obtain the overall equation by reversing half equation 2 and combining with equation 4
- When adding half equations remember to multiply them so each have the same number of electrons
2VO2+ (aq) + 4H+ (aq) + Zn (s) → 2V3+ (aq) + Zn2+ (aq) + 2H2O (l)
Reduction from +3 to +2
- The two half equations we need to consider are 2 and 3
- Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3
- The EΘ value for half equation 2 is more negative than the EΘ for half equation 3
- Zn is the best reducing agent
- V3+ is the best oxidising agent
- We can obtain the overall equation by reversing half equation 2 and combining with equation 3
- When adding half equations remember to multiply them so each have the same number of electrons
2V3+ (aq) + Zn (s) → 2V2+ (aq) + Zn2+ (aq)
Reduction from +2 to 0
- The two half equations we need to consider are 1 and 2
- Vanadium is reduced from an oxidation number of +2 to 0 in half equation 1
- The EΘ value for half equation 1 is more negative than the EΘ for half equation 2
- Zn is not electron releasing with respect to V2+
- This means this reaction is not thermodynamically feasible
Predicting oxidation reactions
- The same method can be used to predict whether a given oxidising agent will oxidise a vanadium species to one with a higher oxidation number
Exam Tip
It is important to not get confused between the two oxo ions of vanadium VO2+ and VO2+
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








