- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.2.12 Autocatalysis
Manganese(II) ions as an Autocatalyst
- Autocatalysis the term used to describe a reaction which is speeded up by a product which acts as a catalyst for the reaction
- If you plot a rate graph of concentration versus time it has an usual shape
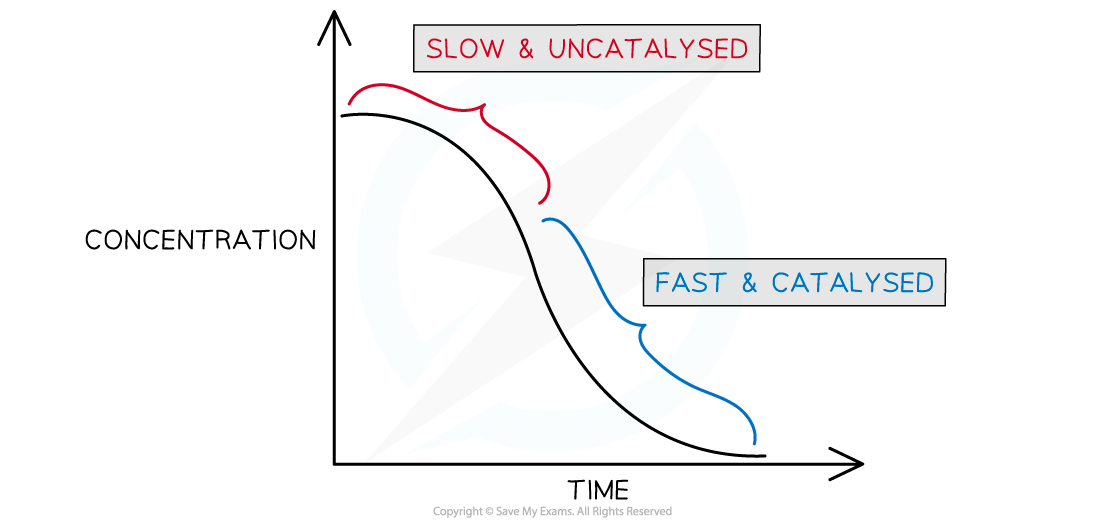
Concentration versus time for an autocatalytic reaction
- The gradient becomes steeper during the course of the reaction which tells you the rate is speeding up, not slowing down over time as the reactants become used up
- An example of an autocatalysed reaction takes place between manganate(VII) ions and oxalate (ethandioate) ions
- The overall equation can be deduced from the half equations
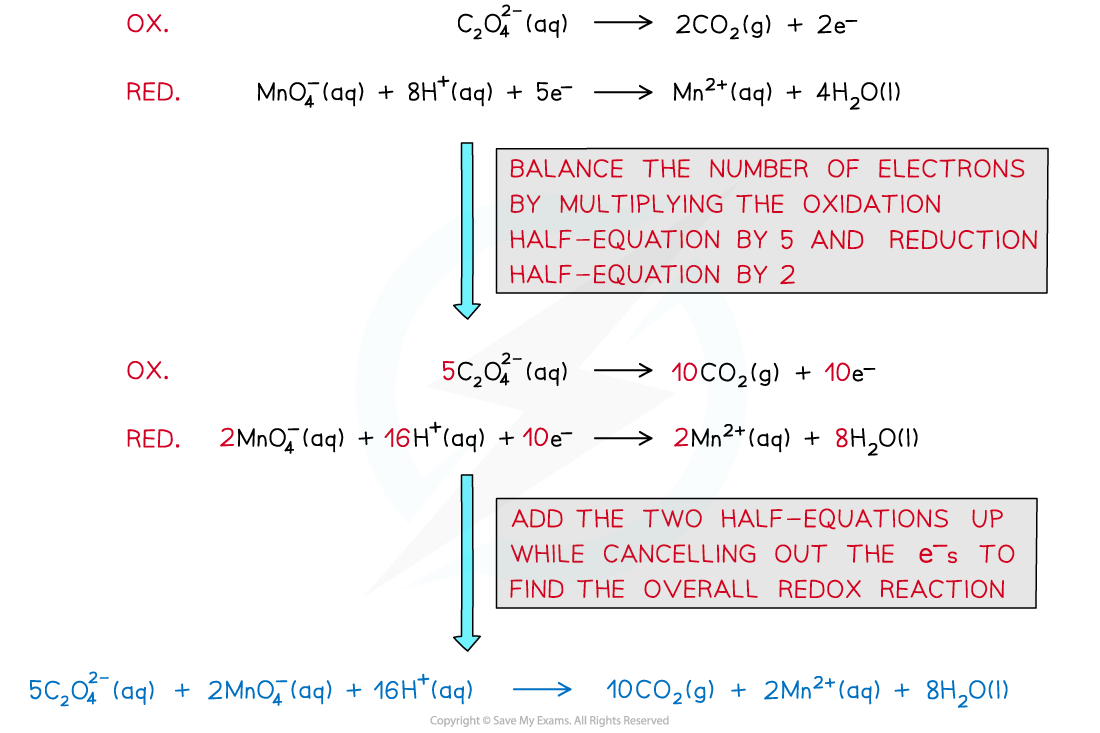
- You can see that one of the products is manganese(II) ions - this is the catalyst
- As more manganese(II) is formed the reaction speeds up
- Like to the role of iron(II) in the previous section, manganese(II) ions take part in a redox cycle between two different oxidation states (+2 → +3 → +2)
4Mn2+ (aq) + MnO4– (aq) + 8H+ (aq) → 5Mn3+ (aq) + 4H2O (aq)
2Mn3+ (aq) + C2O42- (aq) → 2CO2 (g) + 2Mn2+ (aq)
- The manganese(II) is not present in the beginning of the reaction, but as it is formed is speeds up the reaction and is re-generated during the redox cycle
- This reaction is easily followed on a colorimeter as the rate at which the purple manganate(VII) ion is consumed accelerates with time
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









