- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.2.7 Cis-platin
Cis-platin
- Sometimes, complexes with four coordinate bonds may adopt a square planar geometry instead of a tetrahedral one
- Cyanide ions (CN-) are the most common ligands to adopt this geometry
- An example of a square planar complex is cisplatin
- The bond angles in a square planar complex are 90o

Cisplatin is an example of a square planar complex
- In the 1960s the drug cis-platin was discovered, which has been extremely effective in treating a number of different types cancer such as testicular, ovarian, cervical, breast, lung and brain cancer
- Cancer cells grow and replicate much faster than normal cells
- Cis-platin is a square planar molecule that has a geometric isomer with the side groups in different positions

The structures of cis-platin and trans-platin
- The cis-platin works by binding to the nitrogen atoms on the bases in DNA
- The cis-platin passes through the cell membrane and undergoes ligand exchange where the chlorines are replaced by water molecules
- The nitrogen is a better ligand than water and forms dative covalent bonds with the cis-platin
- The cis-platin distorts the shape of the DNA and prevents the DNA from replicating
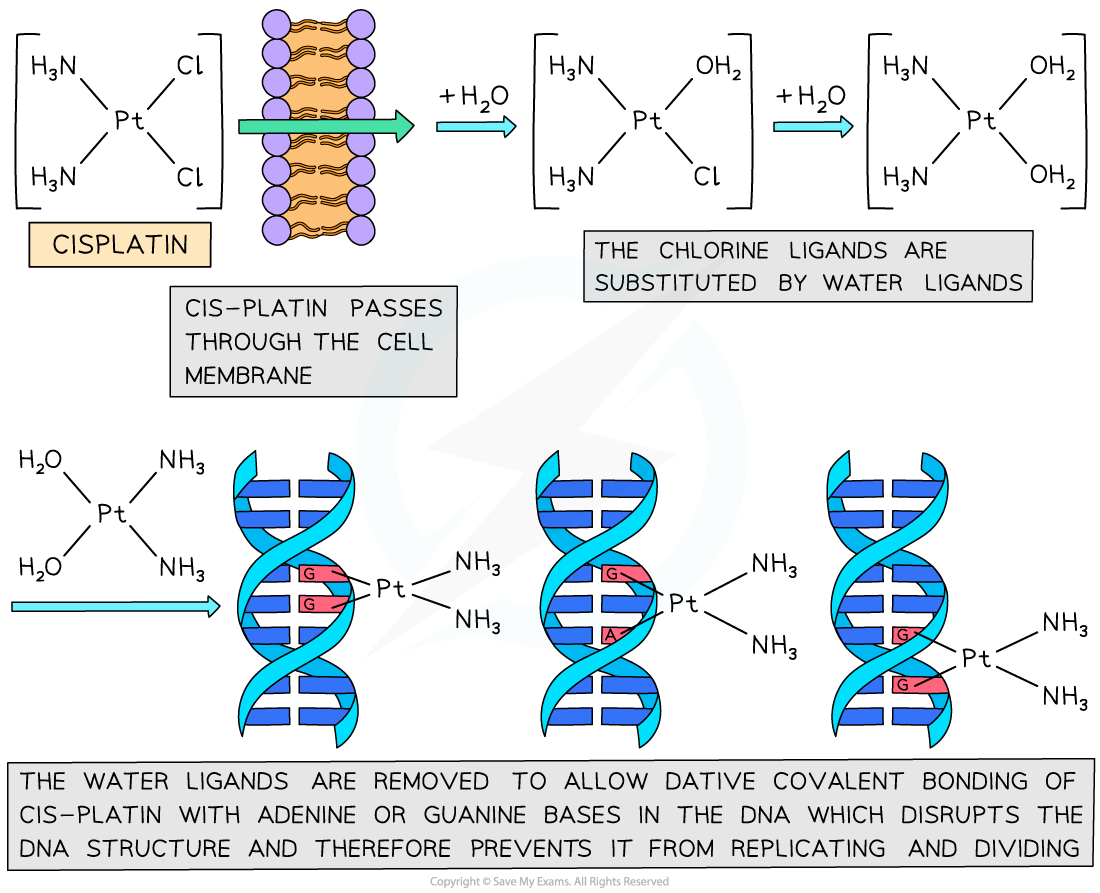
The process by which cis-platin binds to DNA and prevents replication
Adverse Effects
- Cis-platin binds to healthy cells as well as cancerous cells, but affects cancer cells more as they are replicating faster
- Unfortunately, this means that other healthy cells which replicate quickly, such as hair follicles, are also affected by cis-platin
- This is why hair loss is a side-effect of people undergoing cancer treatment
- Despite this drawback, cisplatin is a highly effective drug and society needs to find a balance between the adverse effects of drugs and their therapeutic value
- New therapeutic pathways are constantly under development that aim to deliver drugs that target cancer cells while leaving healthy cells untouched
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








