- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.2.5 Octahedral Complexes
Octahedral Complexes
- Octahedral complexes are formed when a central metal atom or ion forms six coordinate bonds
- This could be six coordinate bonds with six small, monodentate ligands
- Examples of such ligands are water and ammonia molecules and hydroxide and thiocyanate ions
- As there are six ligands, these complexes are sometimes described as having six-fold coordination
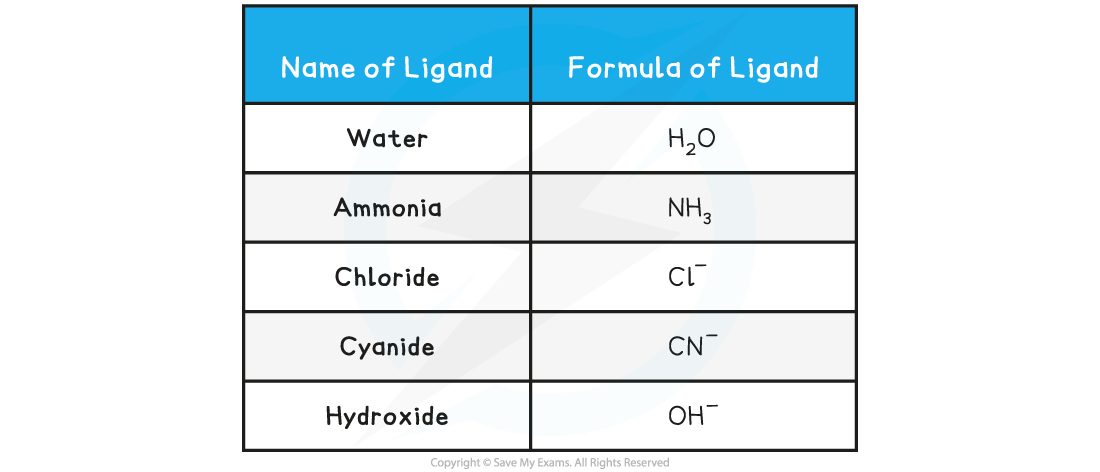
Table showing Examples of Common Monodentate Ligands
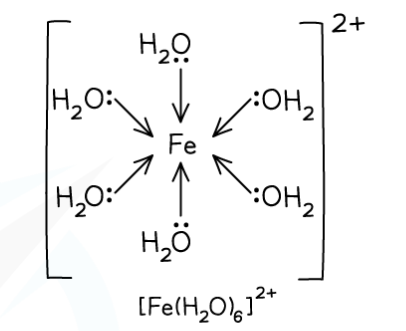
Example of an octahedral complex with monodentate ligands
- It could be six-coordinate bonds with three bidentate ligands
- Each bidentate ligand will form two coordinate bonds, meaning six-coordinate bonds in total
- Examples of these ligands are 1,2-diaminoethane and the ethanedioate ion

Example of an octahedral complex with bidentate ligands
- It could be six-coordinate bonds with one multidentate ligand
- The multidentate ligand, for example, EDTA4-, forms all six-coordinate bonds
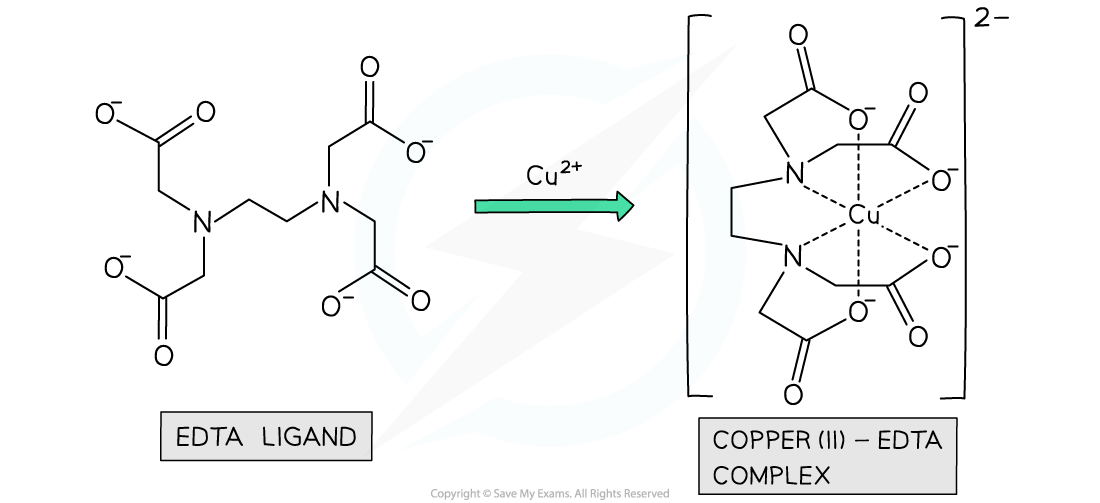
Example of an octahedral complex with a polydentate ligand
- The bond angles in an octahedral complex are 90o
- The coordination number of a complex is the number of dative bonds formed between the central metal ion and the ligands
- Since there are 6 dative bonds, the coordination number for the complex is 6
Exam Tip
Electron pair repulsion theory can be extended to predict and explain the shape of transition metal complexes. The only difference is you should ignore the 3d elctrons in the transition metal ion and overall charge on the complex - just count the number of electron pairs donated by the ligands.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









