- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.1.9 Redox Titration Calculations
Redox Titration Calculations
Redox Titrations
- In a titration, the concentration of a solution is determined by titrating with a solution of known concentration.
- In redox titrations, an oxidising agent is titrated against a reducing agent
- Electrons are transferred from one species to the other
- Indicators are sometimes used to show the endpoint of the titration
- However, most transition metal ions naturally change colour when changing oxidation state
- There are two common redox titrations you should know about manganate(VII) titrations and iodine-thiosulfate titrations
Potassium manganate(VII) titrations
- In these redox titrations the manganate(VII) is the oxidising agent and is reduced to Mn2+(aq)
- The iron is the reducing agent and is oxidised to Fe2+(aq) and the reaction mixture must be acidified, to excess acid is added to the iron(II) ions before the reaction begins
- The choice of acid is important, as it must not react with the manganate(VII) ions, so the acid normally used is dilute sulfuric acid
- As it does not oxidise under these conditions and does not react with the manganate(VII) ions
- You could be asked why other acids are not suitable for this redox titration in the exam so make sure you understand the suitability of dilute sulfuric acid
Table explaining why other acids are not suitable for the redox titration

Indicator and end point
- Potassium permanganate acts as its own indicator, as the purple potassium permanganate solution is added to the titration flask from the burette and reacts rapidly with the Fe2+(aq)
- The burette used in this practical should be one with white numbering not black, as you would struggle to read the values for your titres against the purple colour of the potassium permanganate if black numbering was used
- The manganese(II) ions, Mn2+(aq), have a very pale pink colour but they are present in such a low concentration that the solution looks colourless
- As soon as all of the iron(II), Fe2+(aq), ions have reacted with the added manganate(VII) ions, Mn7+(aq), a pale pink tinge appears in the flask due to an excess of manganate(VII) ions, Mn7+(aq)
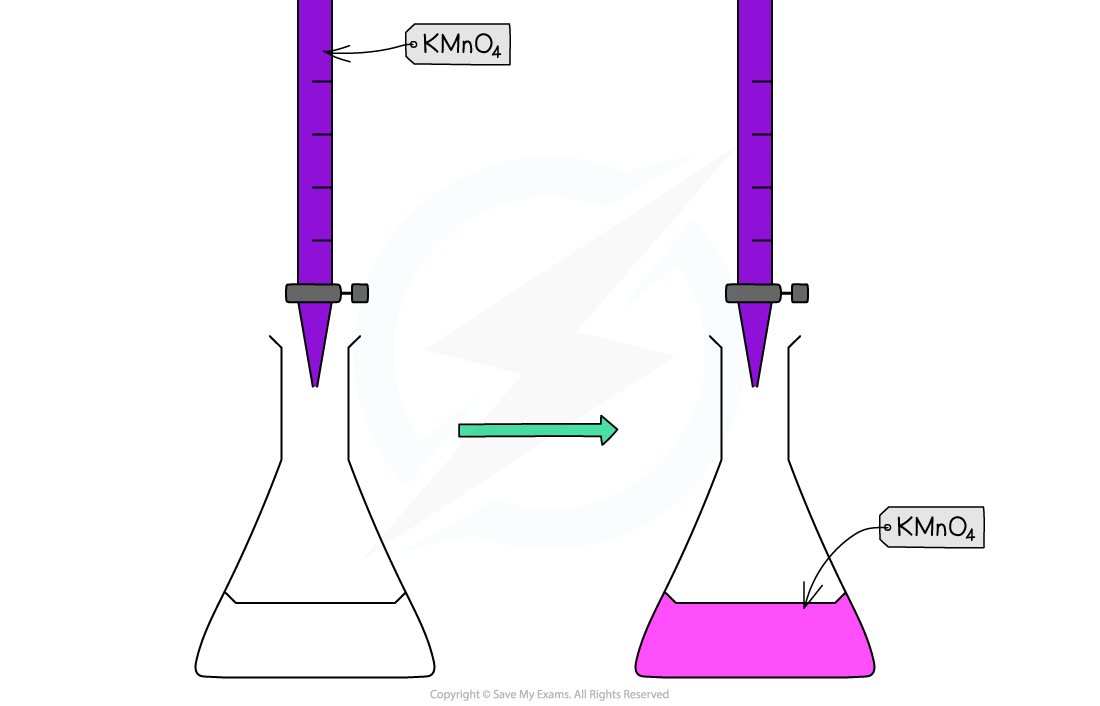
Redox titration colour change for potassium permanganate and iron(II) ions
Worked Example
Equations
Find the stoichiometry for the reaction and complete the two half equations:
MnO4- (aq) + 5e- + 8H+ (aq) → Mn2+ (aq) + 4H2O (l)
Fe2+ (aq) → Fe3+ (aq) + e-
Answers:
Balance the electrons:
MnO4- (aq) + 5e- + 8H+ (aq) → Mn2+ (aq) + 4H2O (l)
5Fe2+ (aq) → 5Fe3+ (aq) + 5e-
Add the two half equations:
MnO4- (aq) + 8H+ (aq) 5Fe2+ (aq) → Mn2+ (aq) + 4H2O (l) + 5Fe3+ (aq)
- Manganate(VII) titrations can be used to determine:
- The percentage purity of iron supplements
- Percentage purity =
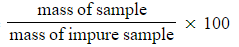
- The formula of a sample of hydrated ethanedioic acid
Worked Example
Analysis of iron tablets
An iron tablet, weighing 0.960 g was dissolved in dilute sulfuric acid. An average titre of 28.50 cm3 of 0.0180 mol dm-3 potassium manganate(VII) solution was needed to reach the endpoint.
What is the percentage by mass of iron in the tablet?
Answer:
-
- MnO4- (aq) + 8H+ (aq) + 5Fe2+ → Mn2+ (aq) + 5Fe3+ (aq) + 4H2O (l)
- 1 : 5 ratio of MnO4- : Fe2+
- Number of moles of MnO4- (aq)
 5.13 x 10-4 moles
5.13 x 10-4 moles - Moles of iron(II) = 5 x 5.13 x 10-4 = 2.565 x 10-3 moles
- Mass of iron(II) = 56.0 x 2.565 x 10-3 = 0.14364 g
- Percentage by mass
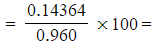 15.0%
15.0%
Iodine-Thiosulfate Titrations
- A redox reaction occurs between iodine and thiosulfate ions:
2S2O32– (aq) + I2 (aq) → 2I–(aq) + S4O62– (aq)
- The light brown/yellow colour of the iodine turns paler as it is converted to colourless iodide ions
- When the solution is a straw colour, starch is added to clarify the end point
- The solution turns blue/black until all the iodine reacts, at which point the colour disappears.
- This titration can be used to determine the concentration of an oxidizing agent, which oxidizes iodide ions to iodine molecules
- The amount of iodine is determined from titration against a known quantity of sodium thiosulfate solution
Worked Example
Analysis of household bleach
Chlorate(I) ions, ClO-, are the active ingredient in many household bleaches.
10.0 cm3 of bleach was made up to 250.0 cm3. 25.0 cm3 of this solution had 10.0 cm3 of 1.0 mol dm-3 potassium iodide and then acidified with 1.0 mol dm-3 hydrochloric acid.
ClO- (aq) + 2I- (aq) + 2H+ (aq) → Cl- (aq) + I2 (aq) + H2O (l)
This was titrated with 0.05 mol dm-3 sodium thiosulfate solution giving an average titre of 25.20 cm3.
2S2O32- (aq) + I2 (aq) → 2I- (aq) + S4O62- (aq)
What is the concentration of chlorate(I) ions in the bleach?
Answer:
-
- One mole of ClO- (aq) produces one mole of I2 (aq) which reacts with two moles of 2S2O32- (aq)
- Therefore, 1 : 2 ratio of ClO- (aq) : S2O32- (aq)
- Number of moles of S2O32- (aq)
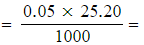 1.26 x 10-3 moles
1.26 x 10-3 moles - Number of moles of I2 (aq) and ClO- (aq) in 25.0 cm3
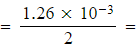 6.30 x 10-4 moles
6.30 x 10-4 moles - Number of moles of ClO- (aq) in 250.0 cm3 = 6.30 x 10-4 x 10 = 6.30 x 10-3 moles
- The 250.0 cm3 was prepared from 10.0 cm3 bleach
- 10 cm3 bleach = 6.30 x 10-3 moles of ClO- ions
- 1.0 dm3 bleach = 0.630 moles of ClO- ions
- Therefore, the concentration of ClO- ions in the bleach is 0.630 mol dm-3
- One mole of ClO- (aq) produces one mole of I2 (aq) which reacts with two moles of 2S2O32- (aq)
Exam Tip
General sequence for redox titration calculations
- Write down the half equations for the oxidant and reductant
- Deduce the overall equation
- Calculate the number of moles of manganate(VII) or dichromate(VI) used
- Calculate the ratio of moles of oxidant to moles of reductant from the overall redox equation
- Calculate the number of moles in the sample solution of the reductant
- Calculate the number of moles in the original solution of reductant
- Determine either the concentration of the original solution or the percentage of reductant in a known quantity of sample
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








