- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记6.1.5 Thermodynamics & Electrode Potential
Thermodynamic Feasibility
Feasibility
- The Eꝋ values of a species indicate how easily they can get oxidised or reduced
- The more positive the value, the easier it is to reduce the species on the left of the half-equation
- The reaction will tend to proceed in the forward direction
- The less positive the value, the easier it is to oxidise the species on the right of the half-equation
- The reaction will tend to proceed in the backward direction
- A reaction is feasible (likely to occur) when the Ecellꝋ is positive
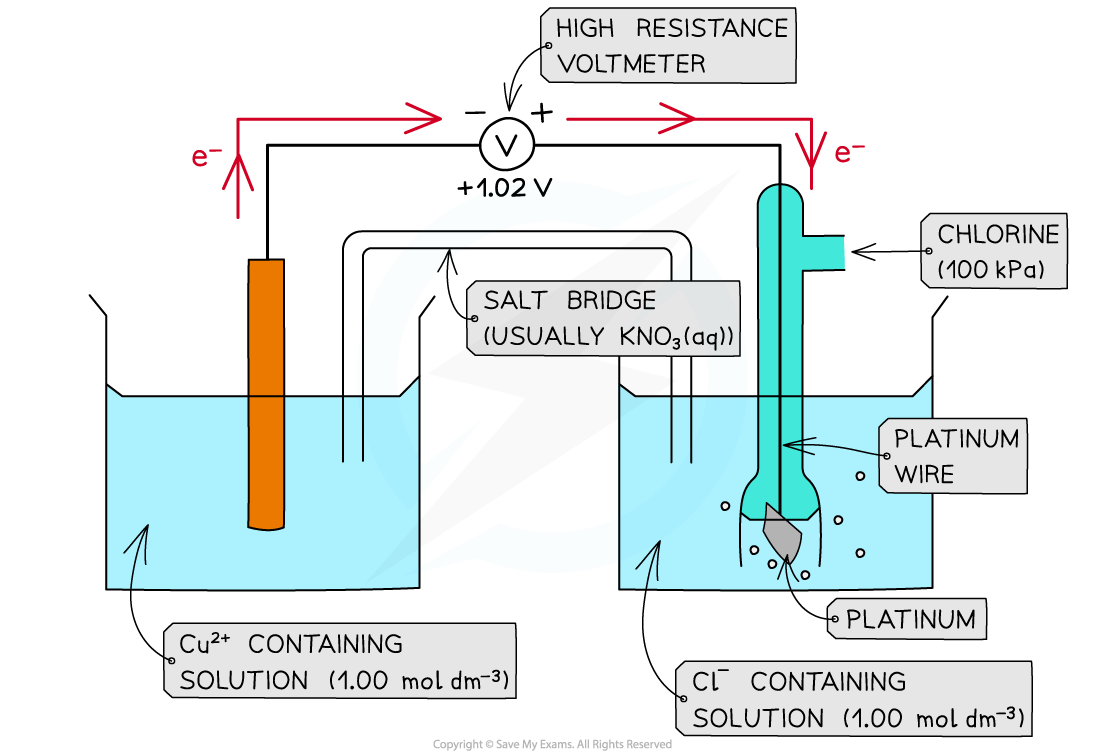
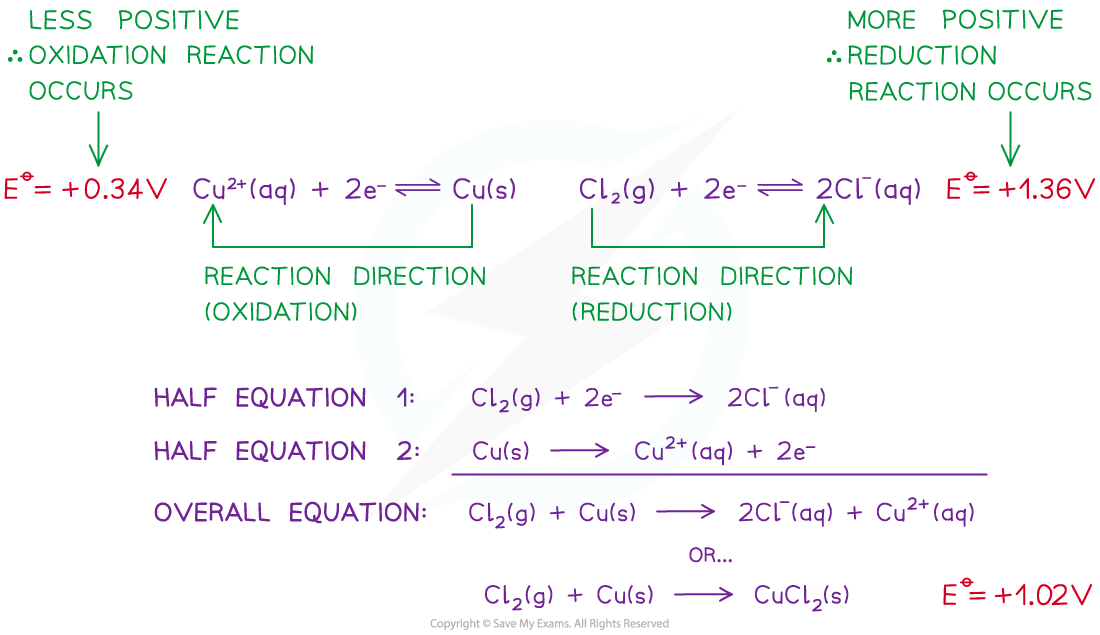
- For example, two half-cells in the following electrochemical cell are:
Cl2 (g) + 2e- ⇌ 2Cl- (aq) Eꝋ = +1.36 V
Cu2+ (aq) + 2e- ⇌ Cu (s) Eꝋ = +0.34 V
- Cl2 molecules are reduced as they have a more positive Eꝋ value
- The chemical reaction that occurs in this half cell is:
Cl2 (g) + 2e- → 2Cl- (aq)
- Cu2+ ions are oxidised as they have a less positive Eꝋ value
- The chemical reaction that occurs in this half cell is:
Cu (s) → Cu2+ (aq) + 2e-
- The overall equation of the electrochemical cell is (after cancelling out the electrons):
Cu (s) + Cl2 (g) → 2Cl- (aq) + Cu2+ (aq)
OR
Cu (s) + Cl2 (g) → CuCl2 (s)
- The forward reaction is feasible (spontaneous) as it has a positive Eꝋ value of +1.02 V ((+1.36) - (+0.34))
- The backward reaction is not feasible (not spontaneous) as it has a negative Eꝋ value of -1.02 ((+0.34) - (+1.36))
A reaction is feasible when the standard cell potential Eꝋ is positive
Exam Tip
You may have to apply your understanding (from the above worked example) to questions with more than 2 equations
- The process is still the same in terms of identifying the most positive / least negative value as the reduction reactions
- This reaction will also contain the oxidising agent on the left hand side
Entropy Change & ln K
- Cell potential is related to both entropy, S, and the equilibrium constant, K
- A larger cell potential means a bigger change in total entropy
- Therefore, we can say that cell potential is directly proportional to total entropy change
Eθ ∝ ΔStotal
- The use of two equations for Gibbs free energy change can also show that:
Eθ ∝ lnK
-
- ΔG = –nF Eθcell
- ΔG = –RT ln K
Exam Tip
You are expected to be aware of the two directly proportional relationships described
However, you are not expected to work with the Faraday based Gibbs free energy equation
ΔG = –nF Eθcell
- The Faraday constant, F, is not given in the Data Booklet
Limitations of Standard Electrode Potential Predictions
- The thermodynamic feasibility of a reaction can be deduced from the electrode potential, however, it gives no information about the rate of reaction
- As standard electrode potentials are measured using solutions, we have to consider the le Châtelier's effect on concentration using non-standard conditions
- For example, the redox equilibrium equation and standard electrode potential for the V3+ | V2+ system are:
V3+ (aq) + e- ⇌ V2+ (aq) Eθ = +0.26 V
-
- If the concentration of V3+ (aq) is greater than 1.0 mol dm-3, then the equilibrium will shift to the right
- This will remove electrons from the system, therefore, making the electrode potential less negative
- If the concentration of V2+ (aq) is greater than 1.0 mol dm-3, then the equilibrium will shift to the left
- This will add electrons to the system, therefore, making the electrode potential more negative
- Any change to the concentration will cause a change to the electrode potential and, therefore, to the overall cell potential
- This is true of any change to the conditions that results in non-standard conditions
- If the concentration of V3+ (aq) is greater than 1.0 mol dm-3, then the equilibrium will shift to the right
- Reaction kinetics can also affect the prediction
- The rate of reaction may simply be so slow that it looks like the reaction isn't happening, when it is
- The reaction may have a high activation energy which inhibits the reaction, for example:
Cu2+ (aq) + 2e- ⇌ Cu (s)Eθ = 0.34 V
2H+ (aq) + 2e- ⇌ H2 (g)Eθ = 0.00 V
-
-
- The reaction of Cu2+ (aq) + H2 (g) → Cu (s) + 2H+ (aq) is thermodynamically feasible with an electromotive force of +0.34 V
- However, this reaction does not occur as the activation energy is so high which means that the reactants are kinetically stable
-
- Another, more basic limitation is the fact that many redox reactions are not aqueous
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








