- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.5.1 Rates of Reaction
Rates of Reaction - Introduction
- The rate of reaction refers to the change in the amount or concentration of a reactant or product per unit time
- The units for rate of reaction are mol dm-3 s-1
- It can be found by measuring:
- The mass lost over time
- The volume of produced over time
- Colour changes, including by the use of colorimetry
- pH changes over time
- Changes in electrical conductivity
- The rate of reaction can be calculated by:
Rate of reaction = 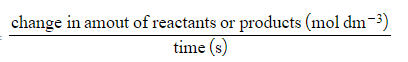
Rate of Reaction
- The following general reaction will be used as an example to study the rate of reaction
D (aq) → E (aq) + F (g)
- The rate of reaction at different concentrations of D is measured and tabulated
Rate of reactions table
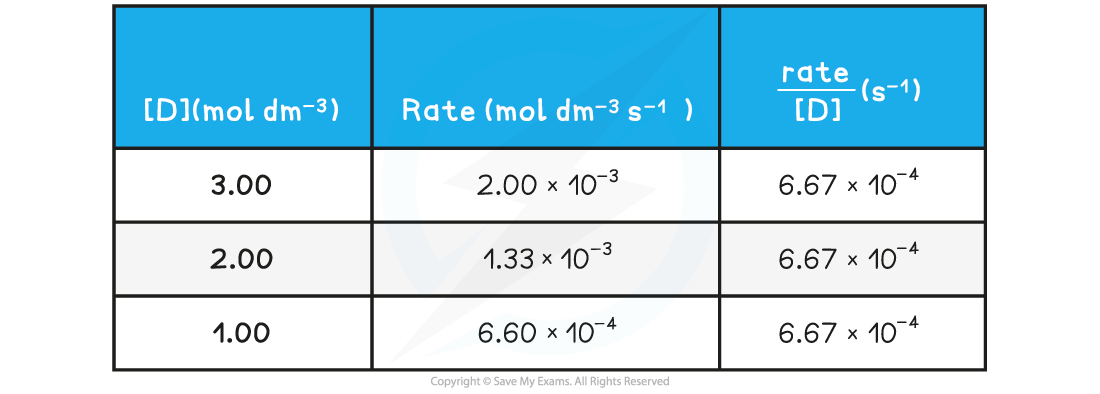
- A directly proportional relationship between the rate of reaction and concentration of D is observed when the results are plotted on a graph:
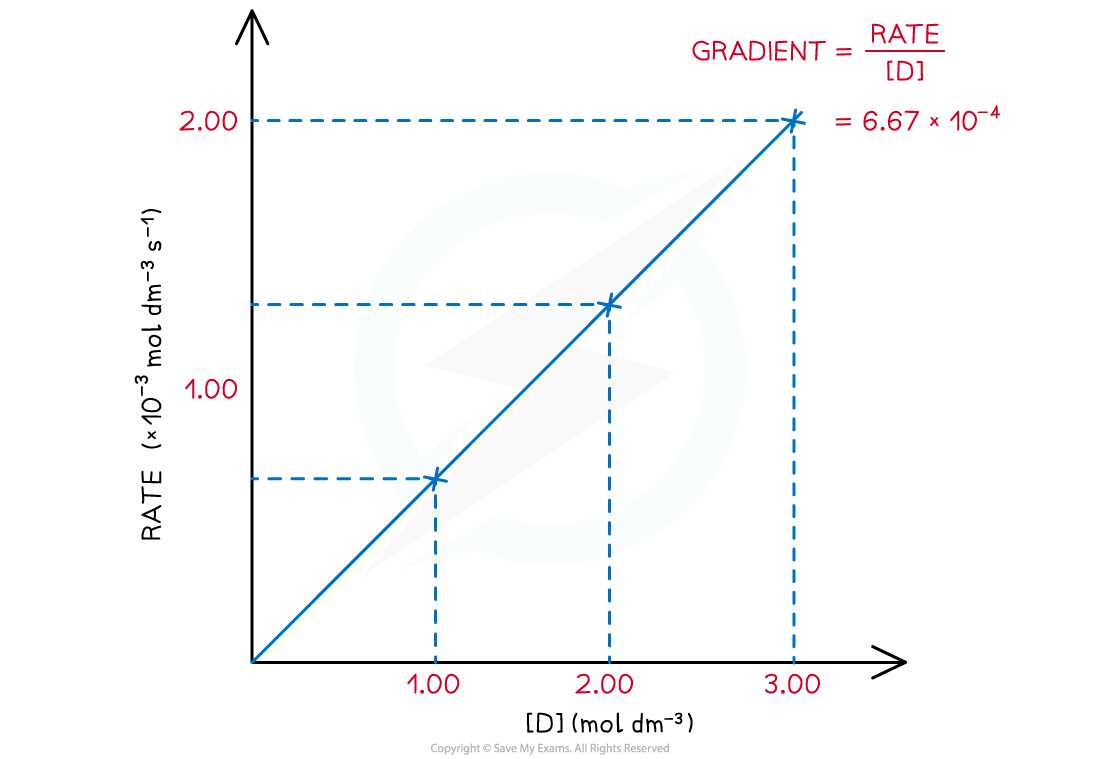
Rate of reaction over various concentrations of D
- This leads to a very common rate expression:
Rate ∝ [D] or Rate = k[D]
- This rate expression means that if the concentration of D is doubled, then the rate doubles
- Equally, if the concentration of D halves, then the rate halves
Rate Equations
- The following reaction will be used to discuss rate equations:
A (aq) + B (aq) → C (aq) + D (g)
- The rate equation for this reaction is:
Rate of reaction = k [A]m [B]n
- Rate equations can only be determined experimentally and cannot be found from the stoichiometric equations
- In the above rate equation:
- [A] and [B] are the concentrations of the reactants
- m and n are orders with respect to each reactant involved in the reaction
- Products and catalysts may feature in rate equations
- Intermediates do not feature in rate equations
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








