- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.4.2 Entropy Calculations
Total Entropy Calculations
- If we take the reaction between sodium and chorine, we know that this is a very exothermic reaction and also involves a decrease in entropy as a solid is produced from a solid and a gas (provided a flame is used to supply the necessary activation energy)
2Na (s) + Cl2 (g) → 2NaCl (s)
- If there is a decrease in entropy, how can the reaction be spontaneous?
- We need to take into account the entropy of the surroundings as well
- The energy being released causes a substantial increase in entropy of the surroundings because there are more ways of arranging the quanta (packets of energy) in the surroundings than the system alone
- Therefore, the total entropy change for a reaction is
ΔSΘ total = ΔS Θsys + ΔSΘsurr
(sys = system and surr = surroundings)
- So, in the case of sodium and chlorine, the large amounts of energy released makes ΔSΘsurr very positive, which will outweigh the negative value of ΔS Θsys
Worked Example
Calculating total entropy change
Calculate the total entropy change in the formation of 1 mole of sodium chloride from its elements in their standard state
ΔSΘsys = -90.1 J K-1 mol-1
ΔSΘsurr = +1379 J K-1 mol-1
Answer
ΔS Θtotal = ΔS Θsys + ΔS Θsurr
ΔSΘ total = -90.1 + 1379 = 1289 J K-1 mol-1
Entropy Change in the System
- Entropy changes are an order of magnitude smaller than enthalpy changes, so entropy is measured in joules rather than kilojoules. The full unit for entropy is J K-1 mol-1
- The standard entropy change (ΔSΘsystem) for a given reaction can be calculated using the standard entropies (Sꝋ ) of the reactants and products
- The equation to calculate the standard entropy change of a system is:
ΔSΘsystem = ΣΔSΘproducts - ΣΔSΘreactants
(where Σ = sum of)
- For example, the standard entropy change for the formation of ammonia (NH3) from nitrogen (N2) and hydrogen (H2) can be calculated using this equation
N2(g) + 3H2(g) ⇋ 2NH3(g)
ΔSΘsystem = (2 x ΔSΘ(NH3)) - (ΔSΘ(N2) + 3 x ΔSΘ(H2))
- Notice that, unlike enthalpy of formation for elements, entropy for elements is not zero and you can find entropy values for elements and compounds in data books
Worked Example
Calculating entropy changes
Calculate the entropy change of the system for the following reaction:
2Mg (s) + O2 (g) → 2MgO (s)
SΘꝋ[Mg(s)] = 32.60 J K-1 mol-1
SΘꝋ[O2(g)] = 205.0 J K-1 mol-1
SΘꝋ[MgO(s)] = 38.20 J K-1 mol-1
Answer
ΔSΘsystem = ΣΔSΘproducts - ΣΔSΘreactants
ΔSΘsystem = (2 x 38.20) - (2 x 32.60 + 205.0)
= -193.8 J K-1 mol-1
Worked Example
Calculating entropy changes
What is the entropy change when ammonia is formed from nitrogen and hydrogen?
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
SΘꝋ[N2 (g)] = 191.6 J K-1 mol–1
SΘꝋ[H2 (g)] = 131 J K-1 mol–1
SΘꝋ[NH3] = 192.3 J K-1 mol–1
Answer:
ΔSΘsystem = ΣΔSΘproducts - ΣΔSΘreactants
ΔSΘsystem= [2 x SΘ(NH3)] - [SΘ(N2)+ (3 x SΘ(H2 ))]
ΔSΘsystem= [2 x 192.3] - [191.6 + (3 x 131)]
ΔSΘsystem = 384.6 - 584.6
ΔSΘsystem= -200 J K-1 mol–1
Exam Tip
Use the stoichiometry of the equation and the correct state of the compounds when calculating the entropy change of a reaction.
Entropy Change in the Surroundings
- To calculate the entropy change of the surroundings, ΔSΘ surr , we need to know the energy that has been transferred to them
- This is given by the enthalpy change, ΔH, and the relationship can be expressed as:
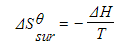
- T is the absolute temperature
- The entropy change of the surroundings depends upon temperature
- The transfer of a given quantity of energy to surroundings at a low temperature will produce a greater entropy change than the transfer of the same amount of energy to the surroundings at a higher temperature
Worked Example
Calculating entropy of surroundings
Calculating entropy of surroundings for the reaction between aluminium oxide and carbon at 298 K
Al2O3 (s) + 3C (s) → 2Al (s) + 3CO (g)
ΔHΘ = +1336 kJ mol-1
T = 298 K
Answer
-
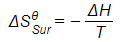
- Convert ΔH from kJ mol-1 to J mol-1 by multiplying by 1000
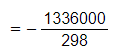
- = - 4483 J K-1 mol-1
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









