- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.3.3 Polarising Power
Polarisation of Ions
- Theoretical lattice energies assume a perfect ionic model where the ions are 100% spherical and the attractions are purely electrostatic
- So overall, there has a been a complete and irreversible exchange of outer shell electrons between the two elements in the compound so that one ion is negatively charged and the other ion positively charged and the charges are whole values
- Theoretical lattice energy can differ from measured lattice energy
- This is because covalent character is introduced when there is polarisation of the anions
- When this occurs, the cation attracts electrons from the anion therefore distorting electron density of the anion
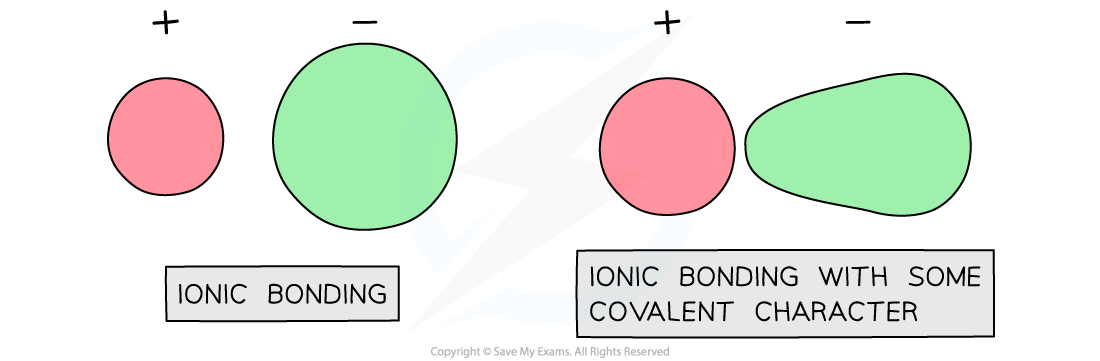 Polarisation of anion leading to covalent character
Polarisation of anion leading to covalent character
Anions & Cations
Cations
- A cation with a large charge density will have a larger polarising power
- The charge density can be calculated as the charge of the cation divided but the surface area, for example
- K+ will have a lower polarising power than Li+ as the ionic radius is greater
- Na+ will have a lower polarising power than Mg2+ as the charge is smaller
- So a cation that is small and highly charged will have the greatest polarising power
Anions
- Anions are polarised
- The polarisability of the anion depends of its ionic radius
- The larger the ionic radius the more easily it will be distorted, for example
- Br- will be more polarisable than Cl- as the ionic radius is larger
转载自savemyexams

在线登记
最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








