- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.2.3 Acid Strength
Acid Dissociation
Strong acids
- A strong acid is an acid that dissociates almost completely in aqueous solutions
- HCl (hydrochloric acid), HNO3 (nitric acid) and H2SO4 (sulfuric acid)
- The position of the equilibrium is so far over to the right that you can represent the reaction as an irreversible reaction
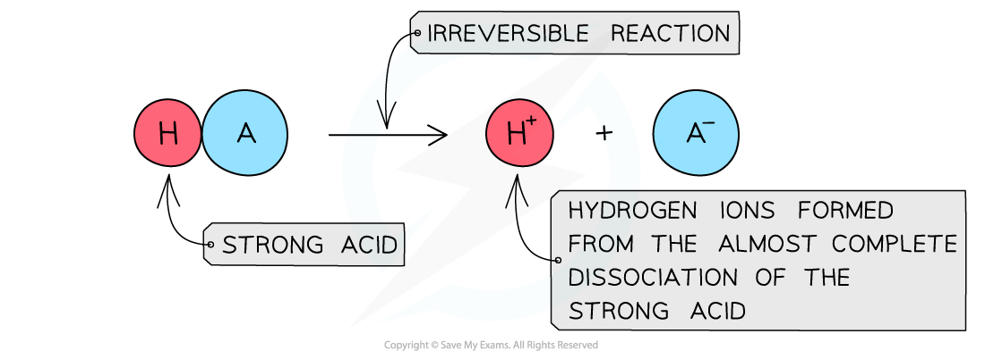
The diagram shows the complete dissociation of a strong acid in aqueous solution
Weak acids
- A weak acid is an acid that partially (or incompletely) dissociates in aqueous solutions
- Eg. most organic acids (ethanoic acid), HCN (hydrocyanic acid), H2S (hydrogen sulfide) and H2CO3 (carbonic acid)
- The position of the equilibrium is more over to the left and an equilibrium is established
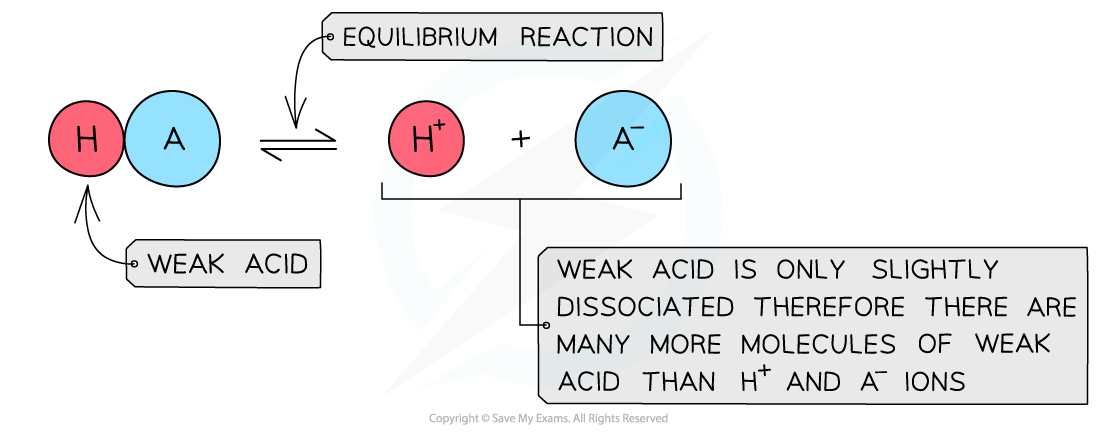
The diagram shows the partial dissociation of a weak acid in aqueous solution
Ka Expressions
- For weak acids as there is an equilibrium we can write an equilibrium constant expression for the reaction

- This constant is called the acid dissociation constant, Ka, and has the units mol dm-3
- Values of Ka are very small, for example for ethanoic acid Ka = 1.74 x 10-5 mol dm-3
- When writing the equilibrium expression for weak acids, the following assumptions are made:
- The concentration of hydrogen ions due to the ionisation of water is negligible
- The value of Ka indicates the extent of dissociation
- The higher the value of Ka the more dissociated the acid and the stronger it is
- The lower the value of Ka the weaker the acid
pH Calculations for Acids
Strong acids
- Strong acids are completely ionised in solution
HA (aq) → H+ (aq) + A- (aq)
- Therefore, the concentration of hydrogen ions, H+, is equal to the concentration of acid, HA
- The number of hydrogen ions formed from the ionisation of water is very small relative to the [H+] due to ionisation of the strong acid and can therefore be neglected
- The total [H+] is therefore the same as the [HA]
Worked Example
What is the pH of 0.01 mol dm-3 hydrochloric acid?
Answer
-
- [HCl] = [H+] = 0.01 mol dm-3
- pH = - log[H+]
- pH = - log[0.01] = 2.00
The pH of dibasic acids
- Dibasic or diprotic acids have two replaceable protons and will react in a 1:2 ratio with bases
- Sulfuric acid is an example
H2SO4 (aq) + 2NaOH (aq) → Na2SO4 (aq) + 2H2O (l)
- You might think that being a strong acid it is fully ionised so the concentration of the hydrogen is double the concentration of the acid
- This would mean that 0.1 mol dm-3 would be 0.2 mol dm-3 in [H+] and have a pH of 0.69
- However, measurements of the pH of 0.1 mol dm-3 sulfuric acid show that it is actually about pH 0.98, which indicates it is not fully ionised
- The ionisation of sulfuric acid occurs in two steps
H2SO4 → HSO4- + H+
HSO4- ⇌ SO42- + H+
- Although the first step is thought to be fully ionised, the second step is suppressed by the abundance of hydrogen ions from the first step creating an equilibrium
- The result is that the hydrogen ion concentration is less than double the acid concentration
Weak acids
- The pH of weak acids can be calculated when the following is known:
- The concentration of the acid
- The Ka value of the acid
- From the Ka expression we can see that there are three variables:

- However, the equilibrium concentration of [H+] and [A-] will be the same since one molecule of HA dissociates into one of each ion
- This means you can simplify and re-arrange the expression to
Ka x [HA] = [H+]2
[H+]2 = Ka x [HA]
- Taking the square roots of each side
[H+] = √(Ka x [HA])
- Then take the negative logs
pH = -log[H+] = -log√(Ka x [HA])
Worked Example
pH calculations of weak acids
Calculate the pH of 0.100 mol dm-3 ethanoic acid at 298 k with a Ka value of 1.74 × 10-5 mol dm-3
Answer
Ethanoic acid is a weak acid which ionises as follows:
CH3COOH (aq) ⇌ H+ (aq) + CH3COO- (aq)
Step 1: Write down the equilibrium expression to find Ka
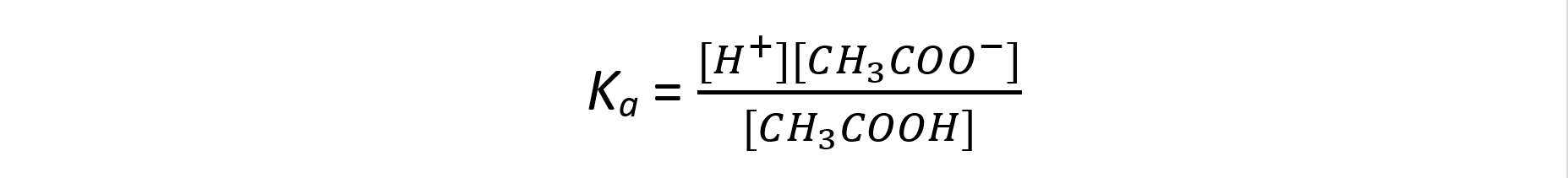
Step 2: Simplify the expression
The ratio of H+ to CH3COO- ions is 1:1
The concentration of H+ and CH3COO- ions are therefore the same
The expression can be simplified to:
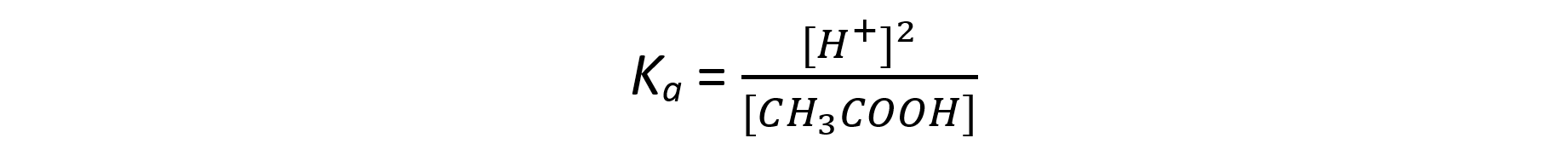
Step 3: Rearrange the expression to find [H+]
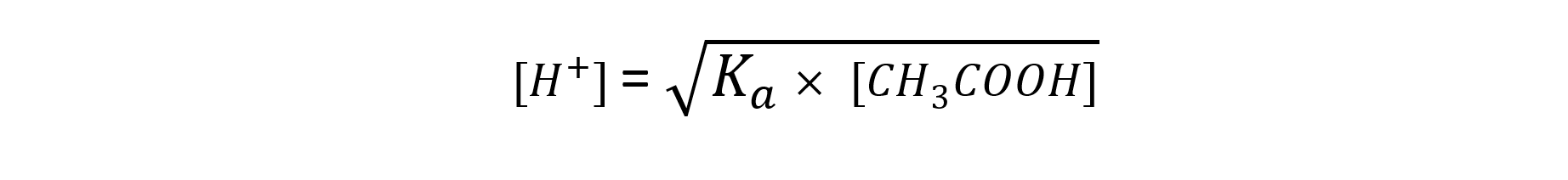
Step 4: Substitute the values into the expression to find [H+]
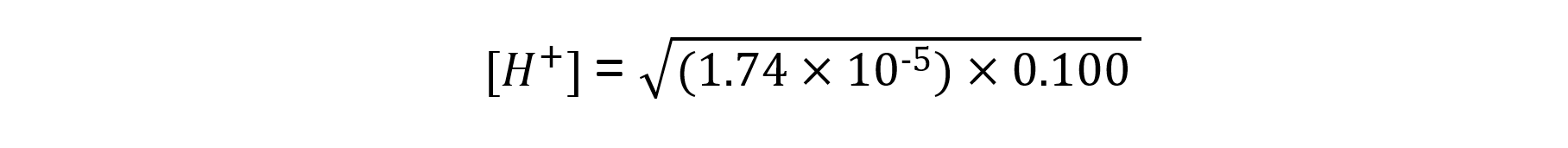
= 1.32 x 10-3 mol dm-3
Step 5: Find the pH
pH = -log[H+]
= -log(1.32 x 10-3)
= 2.88
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









