- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.2.2 pH
Defining pH
- The acidity of an aqueous solution depends on the number of H+ ions in solution
- The pH is defined as:
pH = -log[H+]
-
- where [H+] is the concentration of hydrogen ions in mol dm–3
- Similarly, the concentration of H+ of a solution can be calculated if the pH is known by rearranging the above equation to:
[H+] = 10-pH
- The pH scale is a logarithmic scale with base 10
- This means that each value is 10 times the value below it. For example, pH 5 is 10 times more acidic than pH 6.
- pH values are usually given to 2 decimal places
- The relationship between concentration is easily seen on the following table
pH & [H+] Table
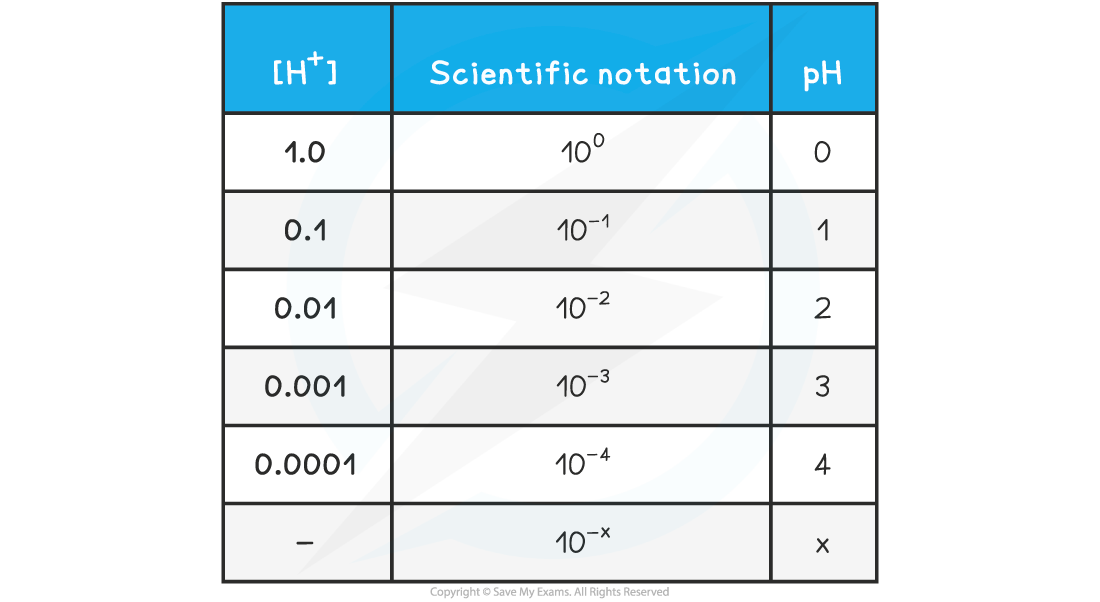
pH & Hydrogen Ion Concentration
Worked Example
pH and H+ calculations
Question 1: Find the pH when the hydrogen concentration is 1.60 x 10-4 mol dm-3
Question 2: Find the hydrogen concentration when the pH is 3.10
Answer
Answer 1:
The pH of the solution is:
pH = -log[H+]
= -log 1.6 x 10-4
= 3.80
Answer 2:
The hydrogen concentration can be calculated by rearranging the equation for pH
pH = -log[H+]
[H+] = 10-pH
= 10-3.10
= 7.94 x 10-4 mol dm-3
Worked Example
Powers of 10
10.0 cm3 of an aqueous solution of an acid of pH = 1.0 is mixed with 990.0 cm3 of distilled water. What is the pH of the final solution?
A. 1
B. 2
C. 3
D. 10
Answer
The correct option is C.
-
- The total volume after dilution is 1000.0 cm3 so the concentration of H+ has been reduced by a factor of 100 or 10-2, which means an increase of 2 pH units
- The final solution is therefore pH 3
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








