- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.1.3 Reaction Conditions & The Equilibrium Constant
Temperature & the Equilibrium Constant
- Changes in temperature change the equilibrium constants Kc and Kp
- For an endothermic reaction such as:
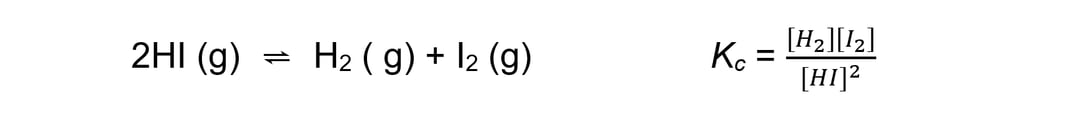
An increase in temperature:
[H2] and [I2] increases
[HI] decreases
Because [H2] and [I2] are increasing and [HI] is decreasing, the equilibrium constant increases
- For an exothermic reaction such as:
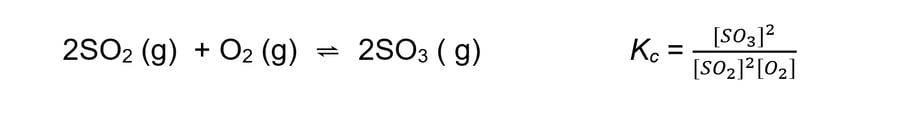
An increase in temperature:
[SO3] decreases
[SO2] and [O2] increases
Because [SO3] decreases and [SO2] and [O2] increases the equilibrium constant decreases
Worked Example
Factors which increase Kp value:
What will increase the value of Kp of the following equilibrium?
2A (g) + B (g) ⇌ 2C (g) ΔH = +6.5 kJ mol-1
Answer
-
- Only temperature changes permanently affect the value of Kp
- An increase in temperature shifts the reaction in favour of the products.
- The [ products ] increases and [ reactants ] decreases, therefore, the Kp value increases.
Temperature & the Equilibrium Position
- How the equilibrium shifts with temperature changes:
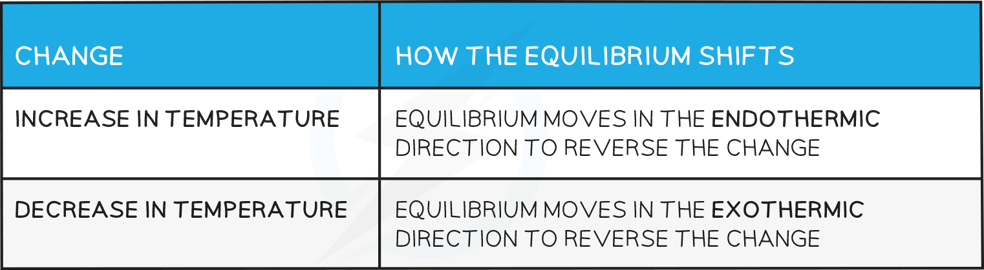
Effect on the value of the equilibrium constant
- For a reaction that is exothermic in the forward direction, increasing the temperature pushes the equilibrium from right to left
- Therefore, the value of the equilibrium constant will decrease as the ratio of [ products ] to [ reactants ] decreases
- Conversely, if the temperature is raised in an endothermic reaction, the value of the equilibrium constant will increase
Changing Reaction Conditions
- If all other conditions stay the same, the equilibrium constant Kc is not affected by any changes in concentration of the reactants or products
- For example, the decomposition of hydrogen iodide:
2HI ⇌ H2 + I2
The equilibrium expression is:
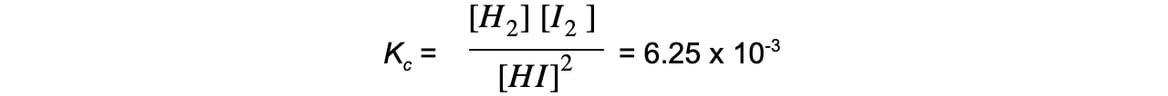
Adding more HI makes the ratio of [ products ] to [ reactants ] smaller
To restore equilibrium, [H2] and [I2] increases and [HI] decreases
Equilibrium is restored when the ratio is 6.25 x 10-3 again
Changes in pressure
- A change in pressure only changes the position of the equilibrium
- If all other conditions stay the same, the equilibrium constant Kc is not affected by any changes in pressure of the reactants and products
- The value of Kp is not affected by any changes in pressure.
- Changes in pressure cause a shift in the position of equilibrium to a new position which restores the value of Kp
- This is analogous to what happens to Kc when you change concentration in an aqueous equilibrium; a shift restores equilibrium to a new position maintaining Kc
Presence of a catalyst
- If all other conditions stay the same, the equilibrium constants Kp and Kc are not affected by the presence of a catalyst
- A catalyst speeds up both the forward and reverse reactions at the same rate so the ratio of [ products ] to [ reactants ] remains unchanged
- Catalysts only cause a reaction to reach equilibrium faster
- Catalysts therefore have no effect on the position of the equilibrium once this is reached
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








