- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记5.1.2 Equilbrium Constant Calculations
Equilibrium Constant Calculations
Calculations involving Kc
- In the equilibrium expression each figure within a square bracket represents the concentration in mol dm-3
- The units of Kc therefore depend on the form of the equilibrium expression
- Some questions give the number of moles of each of the reactants and products at equilibrium together with the volume of the reaction mixture
- The concentrations of the reactants and products can then be calculated from the number of moles and total volume

Equation to calculate concentration from number of moles and volume
Worked Example
Calculating Kc of ethanoic acid
Ethanoic acid and ethanol react according to the following equation:
CH3COOH (I) + C2H5OH (I) ⇌ CH3COOC2H5 (I) + H2O (I)
At equilibrium, 500 cm3 of the reaction mixture contained 0.235 mol of ethanoic acid and 0.035 mol of ethanol together with 0.182 mol of ethyl ethanoate and 0.182 mol of water.
Calculate a value of Kc for this reaction
Answer
- Step 1: Calculate the concentrations of the reactants and products
- [CH3COOH]
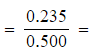 0.470 mol dm-3
0.470 mol dm-3 - [C2H5OH]
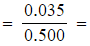 0.070 mol dm-3
0.070 mol dm-3 - [CH3COOC2H5]
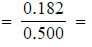 0.364 mol dm-3
0.364 mol dm-3 - [H2O]
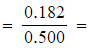 0.364 mol dm-3
0.364 mol dm-3
- [CH3COOH]
- Step 2: Write out the balanced chemical equation with the concentrations of beneath each substance
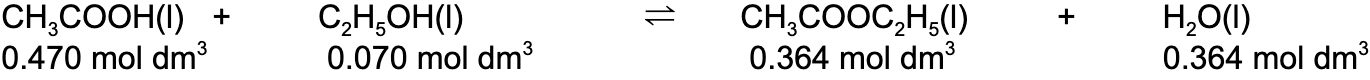
- Step 3: Write the equilibrium constant for this reaction in terms of concentration
Kc =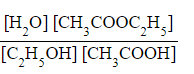
- Step 4: Substitute the equilibrium concentrations into the expression
Kc 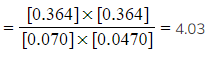
- Step 5: Deduce the correct units for Kc
Kc =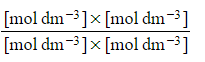
All units cancel out
Therefore, Kc = 4.03
- Note that the smallest number of significant figures used in the question is 3, so the final answer should also be given to 3 significant figures
- Some questions give the initial and equilibrium concentrations of the reactants but products
- An initial, change and equilibrium table should be used to determine the equilibrium concentration of the products using the molar ratio of reactants and products in the stoichiometric equation
Worked Example
Calculating Kc of ethyl ethanoate
Ethyl ethanoate is hydrolysed by water:
CH3COOC2H5(I) + H2O(I) ⇌ CH3COOH(I) + C2H5OH(I)
0.1000 mol of ethyl ethanoate are added to 0.1000 mol of water. A little acid catalyst is added and the mixture made up to 1dm3. At equilibrium 0.0654 mol of water are present. Use this data to calculate a value of Kc for this reaction.
Answer
-
- Step 1: Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and equilibrium table
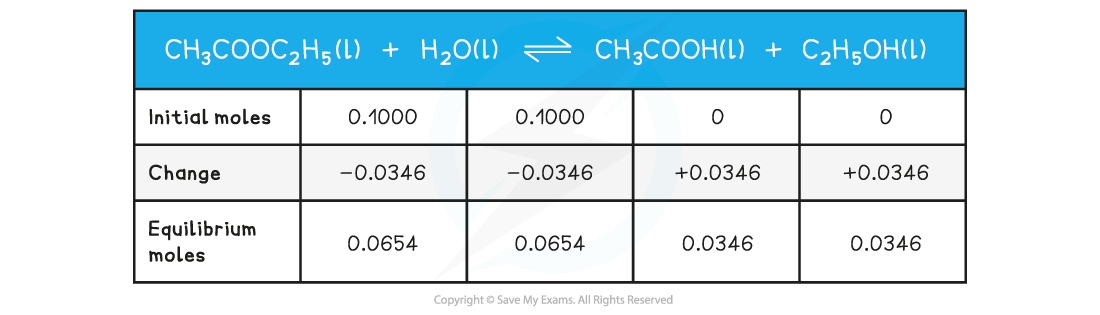
-
- Step 2: Calculate the concentrations of the reactants and products
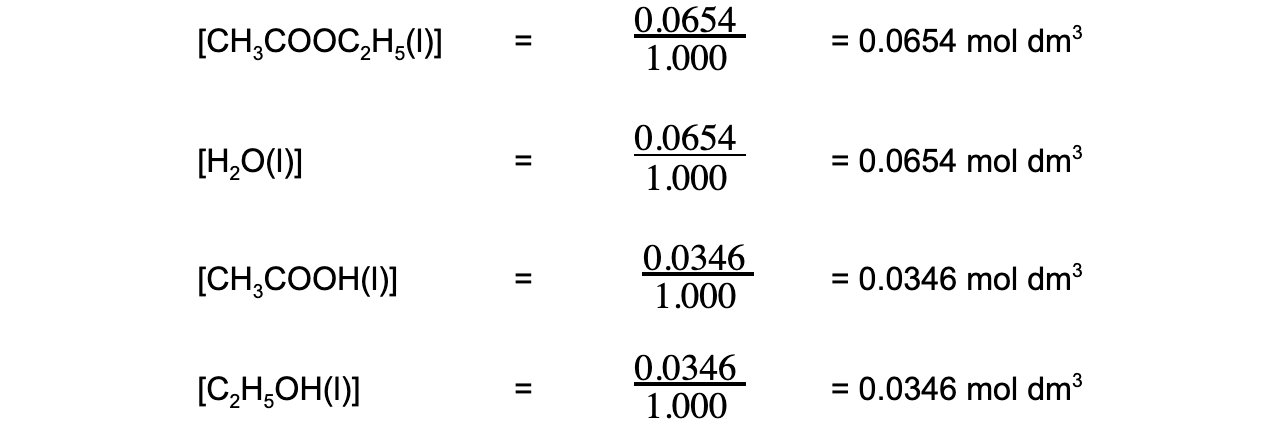
-
- Step 3: Write the equilibrium constant for this reaction in terms of concentration
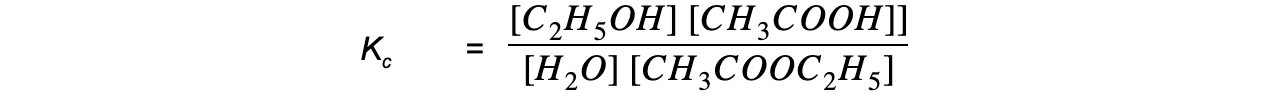
-
- Step 4: Substitute the equilibrium concentrations into the expression
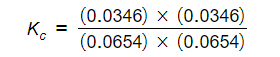
Kc = 0.28
-
- Step 5: Deduce the correct units for Kc

All units cancel out
Therefore, Kc = 0.288
Calculations involving Kp
- In the equilibrium expression the p represent the partial pressure of the reactants and products in Pa
- The units of Kp therefore depend on the form of the equilibrium expression
Worked Example
Calculating Kp of a gaseous reaction:
In the reaction:
2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
the equilibrium partial pressures at constant temperature are
SO2 = 1.0 × 106 Pa, O2 = 7.0 × 106 Pa, SO3 = 8.0 × 106 Pa
Calculate the value for Kp for this reaction.
Answer
-
- Step 1: Write the equilibrium constant for the reaction in terms of partial pressures
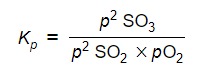
-
- Step 2: Substitute the equilibrium concentrations into the expression

Kp = 9.1 x 10-6
-
- Step 3: Deduce the correct units of Kp
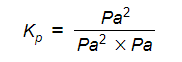
The units of Kp are Pa-1
Therefore, Kp = 9.1 x 10-6 Pa-1
- Some questions only give the number of moles of gases present and the total pressure
- The number of moles of each gas should be used to first calculate the mole fractions
- The mole fractions are then used to calculate the partial pressures
- The values of the partial pressures are then substituted in the equilibrium expression
Worked Example
Calculating Kp of a hydrogen iodide equilibrium reaction:
The equilibrium between hydrogen, iodine and hydrogen iodide at 600 K is as follows:
H2 (g) + I2 (g) ⇌ 2HI (g)
At equilibrium the number of moles present are:
H2 = 1.71 × 10-3
I2 = 2.91 × 10-3
HI = 1.65 × 10-2
The total pressure is 100 kPa.
Calculate the value of Kp for this reaction.
Answer
-
- Step 1: Calculate the total number of moles
Total number of moles = 1.71 x 10-3 + 2.91 x 10-3 + 1.65 x 10-2
= 2.112 x 10-2
-
- Step 2: Calculate the mole fraction of each gas
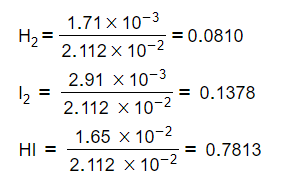
-
- Step 3: Calculate the partial pressure of each gas
H2 = 0.0810 x 100 = 8.10 kPa
I2 = 0.1378 x 100 = 13.78 kPa
HI = 0.7813 x 100 = 78.13 kPa
-
- Step 4: Write the equilibrium constant in terms of partial pressure
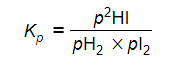
-
- Step 5: Substitute the values into the equilibrium expression
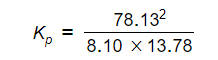
Kp = 54.7
-
- Step 6: Deduce the correct units for Kp
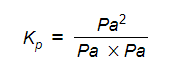
All units cancel out
Therefore, Kp = 54.7
- Other questions related to equilibrium expressions may involve calculating quantities present at equilibrium given appropriate data
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








